Introduction
A body, when receiving a certain amount of thermal energy, can vary its temperature or its physical state, that is, solid, liquid and gas. The temperature variation occurs due to the increased agitation of the particles that make up the body, in this case the amount of heat that the body received is called sensible heat. If the body changes its physical state at constant temperature, we say that the amount of heat is called latent heat. But what is heat? How is it transferred?
Heat is thermal energy in transit, which is determined by the difference in temperature between the systems involved. Heat flows from the higher temperature body to the lower temperature body and can be transferred from one body to another in the following ways: conduction, convection and irradiation. The term heat is used to indicate the transferred energy. Indicated by the letter Q, the unit of heat in the International System of Units is the joule (J), named after the English physicist James Prescott Joule. However, the most used unit is the calorie (cal), however we can relate it to the joule as follows:
Thermal Capacity and Specific Heat
Assuming that there is no change in physical state, imagine a body that receives a certain amount of energy and changes its temperature. It is called thermal capacity C, also called heat capacity, the quotient between the amount of heat (Q) and the variation in temperature suffered by the body (Δt), see:
Do not stop now... There's more after the advertising ;)
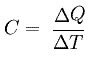
The unit of thermal capacity is the calorie per degree Celsius (cal/°C).
It is defined as specific heat, also called thermal capacity, the numerical value of the amount of heat that causes the variation of one degree Celsius in one gram of the substance. Mathematically, we define it as the ratio between the heat capacity and the mass of the body.

The specific heat unit is cal/g°C.
Fundamental Equation of Calorimetry
Combining the equations that define thermal capacity and specific heat, we can arrive at the equation that provides the amount of sensible heat exchanged by the body of mass m when its temperature changes.
Q = m.c.ΔT
Where c is the specific heat of the substance and ΔT is the temperature variation of the substance.
By Marco Aurélio da Silva
Brazil School Team
See more!!
Calorimeter and heat exchanges
Know what a calorimeter is for.
Thermology - Physics - Brazil School
Would you like to reference this text in a school or academic work? Look:
SANTOS, Marco Aurélio da Silva. "Exchange and Propagation of Heat"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/propagacao-calor.htm. Accessed on June 27, 2021.



