The chemical name of Vitamin C é L-ascorbic acid, or simply Ascorbic acid. This name conveys the chemical and biological roles of this compound. The chemical aspect is that it is acidic, as it contains in its structure a phenolic-hydroxy group. The phenolic group attached to the third carbon in the chain undergoes ionization in aqueous solution, as shown below, releasing the hydroxon ion (H3O+), which is characteristic of acid behavior:
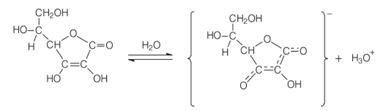
The word “ascorbic” comes from its biological property to fight the disease called scurvy. And the “L” comes from the fact that ascorbic acid has an asymmetric center at carbon 5, having optical activity. However, its anti-scurvy activity derives almost entirely from the L isomer (levogyro), which has a specific rotation in water of 24°.
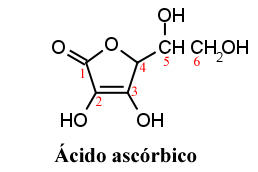
Ascorbic acid was first isolated by Hungarian researcher Szent-Györgi in 1922 as a white crystalline powder.
Humans and other animals such as monkeys, some birds and some fish cannot synthesize vitamin C. The deficiency of this vitamin in the body leads to defective synthesis of collagenous tissue and the aforementioned disease, the
scurvy.Between the main sources of vitamin C, we have fresh fruits, such as cherry, cashew, guava, black currant, mango, orange, acerola, tomato, among others. Potatoes are also a great source of vitamin C, as are peppers and leafy vegetables (bertalha, broccoli, kale, turnips, cassava leaves and yams).

We say "fruits fresh” because vitamin C can be partially or completely destroyed during long periods of storage. For example, every month stored, the potato loses 15% of its vitamin C. Furthermore, heat can also destroy it. Long-cooked foods and foods that have undergone industrial processing contain little vitamin C. In the case of potatoes, if it is cooked without the skin, it will immediately lose 30% to 50% of its property.
One of the main properties of ascorbic acid is its ability to act as a reducing agent. Since it has an exceptional ease of being oxidized in aqueous solution, it is a powerful agent antioxidant, as it can oxidize in place of other compounds.
For example, in everyday life when we cut certain fruits, such as pears, bananas and apples, they darken over time. This is because these fruits contain the enzyme polyphenol oxidase, which causes the enzymatic oxidation of natural phenolic compounds in the presence of molecular oxygen, forming quinones. They polymerize and generate melanins, which are exactly the dark, insoluble pigments we see form in these fruits.
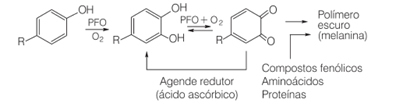
One of the ways to inhibit the action of the polyphenol oxidase enzyme is the addition of ascorbic acid. This is done, for example, when we add orange juice to fruit salad.
In the presence of oxygen and a catalyst, ascorbic acid oxidizes, becoming the dehydroascorbic acid. This acid has a pH below 4, and a lowering of the pH of the fruit tissue causes the browning reaction to slow down. At pH below 3, there is no enzyme activity.
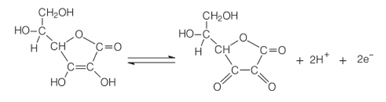
This property of vitamin C is widely used in food industry to prevent the appearance of unpleasant taste, toxicity and for economic reasons, since estimates that around 50% of the loss of tropical fruits in the world is due to the polyphenol enzyme oxidase.
Due to its antioxidant role, vitamin C is also used in cosmetics. Its topical application through these cosmetics allows it to reach levels that are not possible with the oral intake of vitamin C alone. It protects the skin against UV rays and free radicals that lead to premature aging.

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/composicao-aplicacoes-vitamina-c.htm

