O heat and the Thermal energy, motivated by the difference of temperature, which flows from the higher temperature body to the lower temperature body. The energy flow between bodies with different temperatures takes place until reaching the thermal balance, when the temperature of the bodies that exchanged heat becomes equal.

Even if the International System of Units (SI) determine that the unit for energy is joule (J), the most used unit for heat is calorie (cal). One calorie corresponds to the amount of heat needed for 1 g of water to undergo a temperature variation from 14.5 °C to 15.5 °C.
The correspondence between calories and joules is that 1 cal equals approximately 4.18 J.
sensible heat
When a body receives or gives a certain amount of heat, able only to generate temperature variations, without occurring change in the aggregation state of molecules, the heat is called sensitive.
Through the following equation, you can determine the amount of sensible heat received or lost by a body.
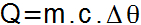
In this equation, the corresponding elements and units of measure are:
Q = Amount of sensible heat (lime);
m = Substance mass (g);
c =specific heat (cal/g°C);
Δθ = Temperature variation (°C or K).
Two observations are important:
O specific heat it is a characteristic quantity of each type of substance that determines the amount of heat needed for 1 g of the element to raise its temperature by 1 °C.
Temperature variations can be expressed both in °C how much in K, because these two scales have 100 intervals, thus presenting the same variations.
Example
Determine the amount of sensible heat needed to heat, from 40 °C to 50 °C, a 4 kg sample of a given substance with a specific heat of 0.5 cal/g °C.
Question data:
Mass m: m = 4 kg = 4000 g
Temperature range: Δθ = 50 – 40 = 10 °C
Specific heat: c = 0.5 cal/g°C
Q = m.c.Δθ
Q = 4000. 0,5. 10
Q = 20,000 cal = 20 kcal
This amount of substance will vary by 10 °C when it receives 20,000 lime of heat.
By Joab Silas
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/fisica/o-que-e-calor-sensivel.htm