John Dalton he formulated an atomic model in 1808 that was able to make sense of some very important concepts within chemistry, such as the concept of molecules, elements and substances. His model, which was proposed from discussions held in Ancient Greece by Democritus and Leucippus, determined that the atom had the following characteristics: it was spherical, massive, indivisible and indestructible.
Dalton associated the atom model to a billiard ball because of its similarity (massive and spherical) with its proposal. Below we have the representation of the atom according to the Dalton model:

Sphere resembling a billiard ball — representation of Dalton's atomic model
O Dalton model is very useful for us to understand some important concepts, such as:
Chemical element: set of atoms that have the same characteristics. In the image below, we have the representation of two chemical elements, since we have two different atoms.

Representation of two different chemical elements using the Dalton model
Molecule: group of atoms. In the image below, we have a representation of a molecule, since we have a group of atoms.

Dalton model used to represent a molecule
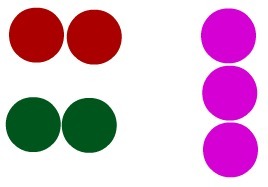
Substance: group of molecules. In the image below, we have a representation of a substance, since we have a group of identical molecules.
Group of identical molecules, according to Dalton's model, that form a substance
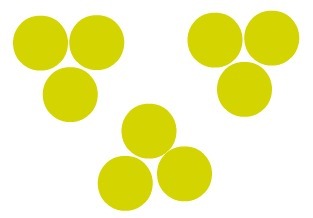
Analyzing the representation below that uses the Dalton's model, we can easily see the amount of atoms, elements, molecules and substances present:
In the image, there are seven atoms, three chemical elements, three molecules and three substances
Do not stop now... There's more after the advertising ;)
atoms: 7 (all spheres);
elements: 3 (red, green, pink);
molecules: 3 (red, green and pink group);
Substances: 3 (red, green and pink group).
Mind Map: Dalton's Atomic Model
* To download the mind map in PDF, Click here!
Dalton's model is still sufficient for us to understand the concept of simple substance. A substance is said to be simple when its molecules are formed exclusively by atoms of the same chemical element. Below we have the representation of three simple substances:
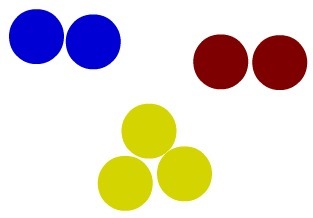
Representation of three simple substances using the Dalton model
Upon analyzing the image, we notice that a molecule is made up only of blue atoms; the other, just for reds; and the last, only by yellow atoms; which denotes that all three are representations of simple substances since they are formed by equal atoms (same element).
If we have in Dalton's model a molecule that has different atoms, we will have the representation of what we call a compound substance, which is nothing more than a substance formed by more than one type of atom, that is, more than one type of chemical element.

Representation of a compound substance according to the Dalton model
Finally, we have five molecules in the image below. By observing them, we will notice that they are all different from each other (different combinations of atoms), containing atoms of different elements. Therefore, in this image we will have five composite substances represented.
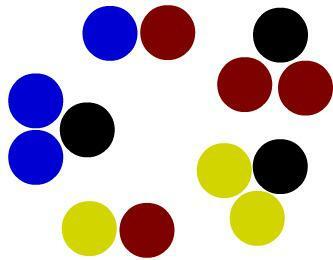
Representation of five different compound substances according to the Dalton model
* Mind Map by Victor Ricardo Ferreira
Chemistry teacher
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Atoms, molecules and substances according to John Dalton"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/atomos-moleculas-substancias-segundo-dalton.htm. Accessed on June 28, 2021.
Chemistry
Atoms and the construction of the Universe, atomic theory, that everything is made, matter is made up of atoms, Theory of the four elements, ancient alchemists, atomic theory, fundamental particle.


