According to the model of orbitals created by Linus Pauling in 1960, the covalent bond that forms the molecules occurs by the fusion or interpenetration of incomplete orbitals of the elements involved in the Link. Thus, it is concluded that if the element has an incomplete orbital (with only one electron), it can only make a covalent bond. If it has two incomplete orbitals, it can make a maximum of two connections and so on.
However, look at the atomic orbitals of the atom of the element carbon, which has the atomic number equal to 6 (Z = 6):
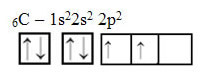
Note that it has two incomplete orbitals, so it should only perform two bindings at most. However, that is not what happens to him. As many know, carbon makes four bonds (it is tetravalent), so this model of orbitals does not explain the case of carbon.
To end this impasse, a new theory was created that better explained this issue: the Hybridization Theory.

This means that hybridization is a “mix” of pure orbitals.
For carbon there are three types of hybridization, which are: sp3, sp2 and sp.
To understand how hybridization occurs, let's look at the first type of carbon hybridization, the sp type.3:
This type of hybridization occurs in the methane molecule (CH4). Note that there are four identical covalent bonds between carbon, which is the central element, and four hydrogens. So, see what the incomplete hydrogen orbital is:
Do not stop now... There's more after the advertising ;)
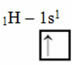
As each hydrogen has an incomplete s-type orbital, it is necessary to receive one more electron, that is, each one makes only one covalent bond with the carbon. That's why carbon needs to have four incomplete orbitals. How does this happen? Through hybridization.
When an electron from the 2s orbital absorbs energy, it passes into the empty 2p orbital. Thus, we say that this jump of the electron from the 2s to the 2p sublevel is a “promotion” of the electron. In this way, the carbon remains in its excited or activated state, with four hybridized orbitals available to carry out the covalent bonds:
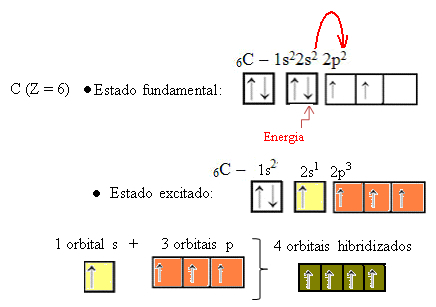
Note that the hybrid orbitals formed are equivalent to each other, but different from the original pure orbitals.
In this way, the bond between the s orbital of each of the four hydrogen atoms occurs with these four hybridized carbon orbitals:
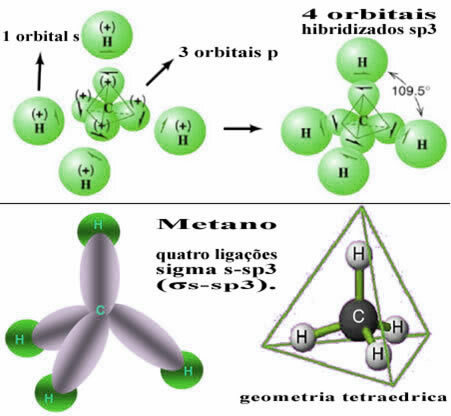
As seen above, the methane molecule has a regular tetrahedron structure, with the four electron clouds at each vertex and adjacent angles of 109°28’. Since the bond was made between an s orbital of each hydrogen and a hybridized sp orbital3 for each connection, then we have that they are four sigma s-sp links3 (σs-sp3).
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Sp3 type hybridization"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/hibridizacao-tipo-sp3.htm. Accessed on June 28, 2021.