It can happen that there are acids of the same element, and this element has the same oxidation number (NOX), but the difference is in the degrees of hydration.
For example, below, we have three acids formed by the element phosphorus (P):
H3DUST4 H4P2O7 HPO3
Note that in all three acids the oxidation number of phosphorus is +5; the difference is in the degree of hydration.
Based on this, these acids are differentiated in the nomenclature through the prefixes ortho, pyro and meta.
The most hydrated acid is called ortho. In the example given, the first (H3DUST4) is called acid orthophosphoric, because it is the most hydrated of the three. The ortho prefix is expendable, so most of the time this acid will just be called phosphoric acid.
The prefixes pyro and meta are used with ortho acid as the reference point:
Do not stop now... There's more after the advertising ;)
- Pyrus: 2 molecules of ortho minus 1 molecule of H2O
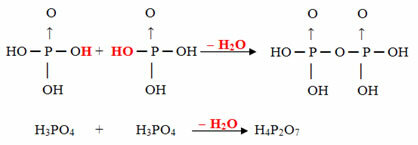
Example: The H4P2O7 is called acid pirophosphoric because it is equal to two molecules of orthophosphoric acid (H3DUST4) minus one water molecule.
2. H3DUST4 =H6P2O8
H6P2O8 - H2O = H4P2O7
This process is a intermolecular dehydration:
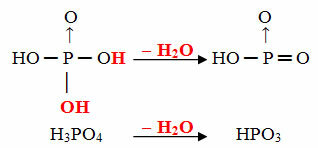
- Goal: 1 molecule of ortho minus 1 molecule of H2O
Example: The HPO3 is called acid goalphosphoric because it is equal to a molecule of orthophosphoric acid (H3DUST4) minus one water molecule.
H3DUST4 - H2O = HPO3
This process is a intramolecular dehydration:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Degree of hydration of acids"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/grau-hidratacao-dos-acidos.htm. Accessed on June 28, 2021.
Orthophosphoric acid has the following molecular formula: H3DUST4. From there, mark the alternative that indicates pyrophosphoric acid and metaphosphoric acid, respectively:
Degree of Ionization, Hydrochloric Acid, Volatility, Acetic Acid, Svante Arrehenius, Acids Conduct Electricity, neutralization reactions, Reaction with carbonates and bicarbonates, red phenolphthalein solution, litmus paper blue.