Some compounds have in their structure double bonds alternating with single bonds. The most famous of them all is benzene, whose structure was proposed in 1865 by the German chemist Friedrich August Kekulé (1829-1896). Its structure would be cyclic and formed by three double bonds interspersed with three single bonds, as shown in the figures below:
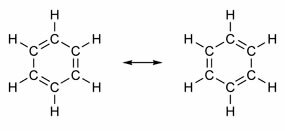
Both ways of representing benzene are acceptable, as it is possible to change the electrons in the π bonds without changing the position of the atoms. However, neither represents exactly what he is nor explains his behavior. It should behave like an alkene and provoke addition reactions, but in practice this does not happen. Benzene is quite stable and acts as if it doesn't have double bonds; it gives substitution reactions like in alkanes.
In 1930, the American scientist Linus Pauling proposed the resonance theory that explained this apparent contradiction. This theory said:
Do not stop now... There's more after the advertising ;)
“Whenever, in a structural formula, we can change the position of the electrons without changing the position of the atoms, the real structure no will be none of the obtained structures, but rather a resonance hybrid of those structures.”
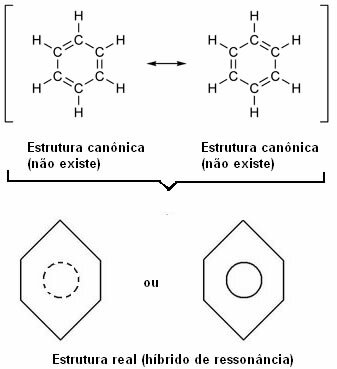
This effect is proven by the size of the carbon bonds, and the distance between them. This distance is intermediate to that of the single bond (1.54 Å) and that of the double bond (1.34 Å); being, therefore, 1.39 Å, due to the resonance effect.
This effect can also be seen in the structure of the ozone molecule (O3), as shown below:
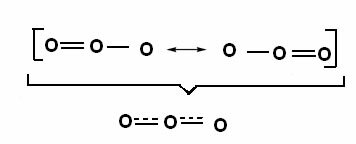
Canonical structures and ozone resonance hybrids.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team.
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Resonance in chemical compounds"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/ressonancia-compostosquimicos.htm. Accessed on June 28, 2021.