Aldehyde it is an organic function that has as its main characteristic the presence of the carbonyl group (C=O)on the edge of the carbon chain. Below is the representation of the carbonyl at the end of a chain:
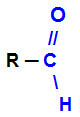
Carbonyl at the end of a chain indicating aldehyde organic function
Whenever the carbonyl is located at the end of a carbon chain, it will have a hydrogen atom attached directly to it. The R group attached to the carbon can be a hydrogen or any radical. Thus, the smallest existing aldehyde has only a single carbon atom:
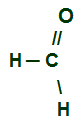
Structural formula of the lowest aldehyde
There is also the possibility that an aldehyde has more than one carbonyl in the chain:
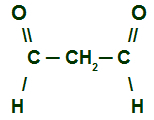
Structural formula of an aldehyde that has two carbonyls
Let's meet now the nomenclature, characteristics and applications of aldehydes:
a) IUPAC Nomenclature
THE rule of nomenclature established by IUPAC for an aldehyde é:
Prefix (indicating the number of carbons) + infix the type of bonds + al
follow up some application examples of the naming rule for a aldehyde:
Example 1: Propanal
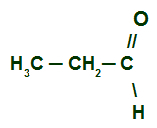
Propanal structural formula
We have an aldehyde of saturated chain (single bonds only) and normal (no branches). So, to name it, just follow the prefix, infix and suffix (al) sequence. He presents three carbons(prop prefix) and simple links (an infix). His name is therefore:
Propanal
Example 2: 2-methyl Butanal
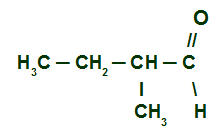
Structural formula of 2-methyl Butanal
here we have a branched and saturated aldehyde (only single bonds), so we must locate the main chain, which must have the carbonyl carbon and the largest number of carbons. The main chain is the horizontal sequence of carbons. Lastly, we number the chain from the carbonyl carbon:
Do not stop now... There's more after the advertising ;)
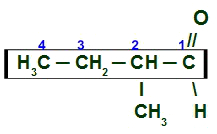
2-Methyl Butanal Main Chain Numbering
To name this aldehyde, we take into account that, on carbon 2, we have the presence of a radicalmethyl (CH3-) and that the main chain has four carbons (prefix but) and just simple calls (infix an). His name is therefore:
2-methyl Butanal
Example 3: 3-ethylhex-4-enal
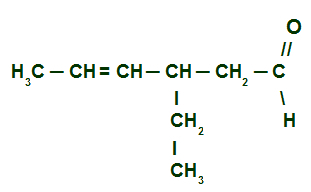
Structural formula of 3-ethylhex-4-enal
here we have a branched and unsaturated aldehyde (has a double bond), so we must locate the main chain, which must have the carbon of the carbonyl and the carbon of the double. The main chain, in this case, will be the horizontal sequence of carbons. Lastly, we number the chain from the carbonyl carbon:
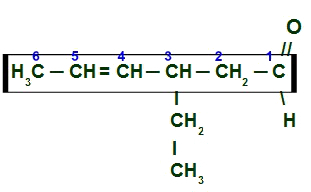
Main chain numbering of 3-ethylhex-4-enal
To name this aldehyde, we take into account that, on carbon 3, we have the presence of a radical ethyl (CH3-CH2) and that the main chain presents six carbons (prefixed hex) and one double bond (prefixed at), located on carbon 4. His name is therefore:
3-ethylhex-4-enal
b) Characteristics of aldehydes
At main features of the aldehydes are:
They are extremely reactive substances;
They have a lower density than water;
The physical state (solid, liquid or gas) depends on the amount of carbon in the aldehyde. Aldehydes that have up to two carbons, for example, are gases;
Most of them have pleasant odors;
Its molecules are polar;
They feature easy combustibility (they burn easily).
c) Applications of aldehydes
Perfume production
Plastics production
Pharmaceutical industry
Food industry
As an industrial solvent
Mirror production
Disinfectant production
resin production
Insecticide production
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is aldehyde?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-aldeido.htm. Accessed on June 28, 2021.
Chemistry

Aldehydes, carbonyl compounds, carbonyl group, Main aldehydes, Ethanal, raw material in the pesticide and drug industry, Metanal, formaldehyde, plastic and resin industry.