Decantation is a physical process for separating heterogeneous liquid-solid and liquid-liquid mixtures. It is based on the difference in density between its components and the fact that they are insoluble in each other.
Decantation is related to the sedimentation that occurs when the mixture is left to rest for a certain time. The denser component that has its particles in suspension will, over time, by gravity, settle to the bottom of the container. Thus, when this sedimentation ends, decantation takes place, which is simply to tilt the container that contains the mixture and pour the less dense liquid, which is in the part, into another container. from above.
For example, in a mixture of sand and water left to rest, the sand particles will settle down to the bottom of the container, and the water can be separated by decanting:
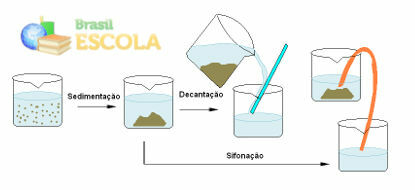
Mixture separation process involving sedimentation, decantation and siphonation
Have you ever heard of wine decanting? This process refers to the passage of red wine from its bottle to a crystal or wine container called a decanter or decanter, which is mainly made to separate the wine from the dregs that are formed and accumulated along the years old. Decanting the wine also allows for oxygenation of the drink, totally releasing the aromas contained in the bottle, which is, therefore, a process that contributes positively to the taste.
Do not stop now... There's more after the advertising ;)

Waiter decanting red wine before being served
Sedimentation can take some time, but it can be accelerated by means of centrifuges that perform a rotating movement; thus, by inertia, the larger particles settle to the bottom. The blood below was centrifuged, and the liquid part of the plasma was separated:

Laboratory centrifuged blood sample
The mentioned separations were of heterogeneous mixtures of the liquid-solid type. In the case of heterogeneous mixtures of the liquid-liquid type, such as oil and water, it is customary to use in the laboratory equipment called bromine funnel or separation funnel. After vigorous shaking, it is left to rest, the denser liquid, which usually remains in the the bottom part is separated by the tap opening at the bottom of the bromine funnel and is placed in another container. When closing the faucet, the less dense liquid remains inside the funnel, being later collected in another container.

Decanting liquids with a bromine funnel or separating funnel
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "What is decanting?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-decantacao.htm. Accessed on June 28, 2021.

