At amides are organic compounds characterized by presence of a nitrogen (N) directly attached to a carbonyl (C=O). These are substances available in a natural way, one of them is in the excreta of mammals (urea), but they can also be obtained by artificial synthesis.
Amides can be produced, for example, by ammonium salt dehydration, process used in the manufacture of polymers. They are also used as fertilizers, due to its availability of nitrogens, and how drug, having antimicrobial action.
Read more:Acrylamide - amide that can be originated from heating some foods
Amide structure
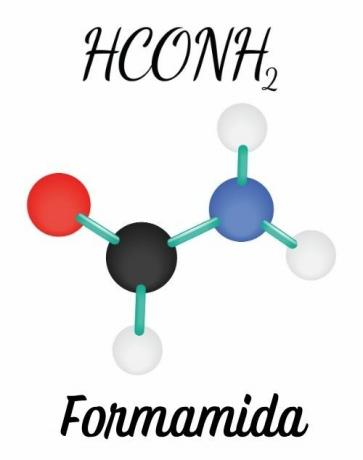
The amides are formed by a nitrogen bonded directly to a carbonyl or acyl group (R-C=O). The double bond between the carbon it's the oxygen and the possibility of moving this pair to nitrogen give the molecule planar geometry, unlike amines, which have pyramidal geometry.
Do not stop now... There's more after the advertising ;)
Classification of amides
Classification according to the number of organic substituents
As well as the amines, amides are classified according to the number of substituting organic radicals that nitrogen has, however, for amides, we have to consider that one of the group's ligands will be the acyl group, that is, we will only have amides of the type:
- Unsubstituted amide: has nitrogen bonded to two hydrogens and a carbonyl group.
Example:

- Monosubstituted amine: has nitrogen bonded to a hydrogen, a carbonyl group and an organic radical. In this case, where one of the hydrogens has been replaced by a carbon chain, consider R as an organic group.
Example:

- Disubstituted amine: has nitrogen bonded to two organic radicals and a carbonyl. In this case, the two hydrogens were replaced by carbon chains.
Example:

See too: How to classify organic halides?
Classification according to the number of carbonyls linked to nitrogen
Amides can also be classified according to the number of carbonyls attached directly to the nitrogen of the molecule.
- Primary amides: only one acyl group linked to nitrogen (R-CO)NH2 .
- secondary amides: two carbonyls or acyl group linked to nitrogen (R-CO)2NH.
- tertiary amides: three acyl groups linked to nitrogen (R-CO)3No.

Nomenclature of amides
THE nomenclature for amides will be given by:
prefix indicating the number of carbons in the chain + location and infix indicating unsaturations (if any) + amide termination |
See the table below:
Prefix (no. of carbons) |
Infix (chain saturation) |
Suffix (functional group) |
|||
1 carbon |
Met- |
Single calls only |
-an- |
amides |
-amide |
2 carbons |
Et- |
||||
3 carbons |
Prop- |
1 double bond |
-en- |
||
4 carbons |
But- |
||||
5 carbons |
pent- |
2 double bonds |
-dien- |
||
6 carbons |
Hex- |
||||
7 carbons |
Hept- |
1 triple bond |
-in- |
||
8 carbons |
Oct- |
||||
9 carbons |
Non- |
2 triple links |
-diin- |
||
10 carbons |
Dec- |
The carbon count must start with the side closest to the nitrogen of the functional group.
Examples:
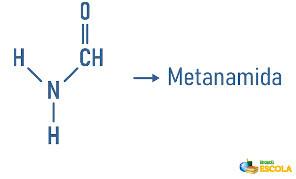



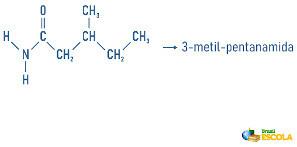
Amines may also receive in their nomenclature a specification on the classification of the molecule:
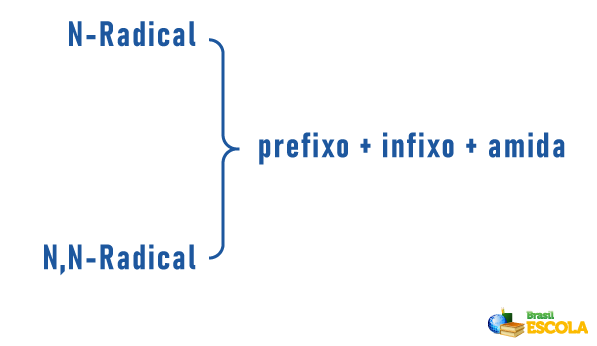
Remembering that nomenclature for radicals is formed by: Prefix indicating number of carbons + termination “il” or “ila”. The radicals are placed in the nomenclature in alphabetical order.
Examples:


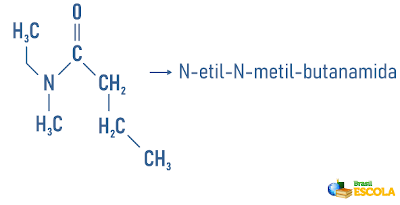
Also access: Nomenclature of cyclic and branched hydrocarbons
Properties of amides
- High melting and boiling point, which will have a scaled value according to the size and spatial arrangement of the carbon chain.
- Highly polar due to the presence of carbonyl and nitrogen.
- Unsubstituted and monosubstituted amides hydrogen bond.
- Smaller and simpler molecules are water soluble. The size of the molecule also affects the solubility of amides: the longer the carbon chain, the less soluble they will be in water.
- The amides have a basic character due to their propensity to receive H ions+.
Application of amides
- Used as intermediaries in the manufacture of polyethylenes such as nylon.
- Applied in the formulation of drugs such as sulfanilamide and penicillin, active principles of bactericidal drugs for infection control.
- Urea, which can be obtained synthetically or as a product excreted by mammals, is a substance of the amide group, a diamide. It is used as a food supplement in agriculture and as a fertilizer.

Obtaining the amides
Amides are easily found in a natural form, but their synthetic form is still widely used in industrial processes. Below are some amide production reactions based on other nitrogenous compounds.
Ammonium salt dehydration reaction

Reaction of amines with acid chloride
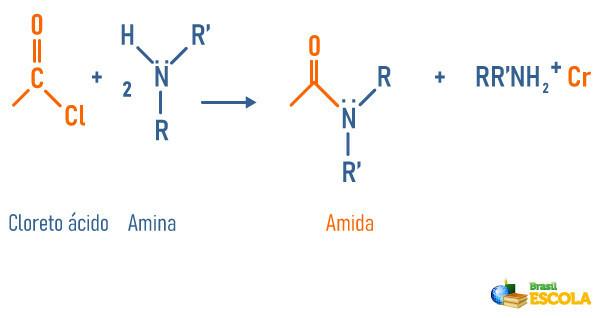
-
Reaction of anhydrides with amines
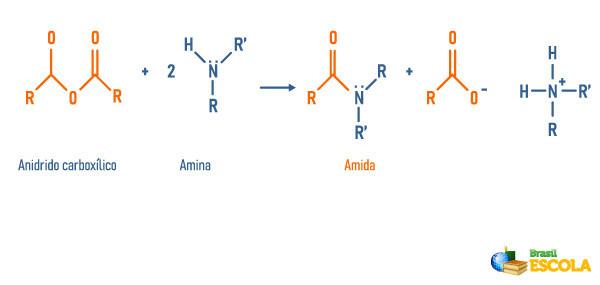
Reaction of esters with amines

Structural rearrangement of an aldoxime
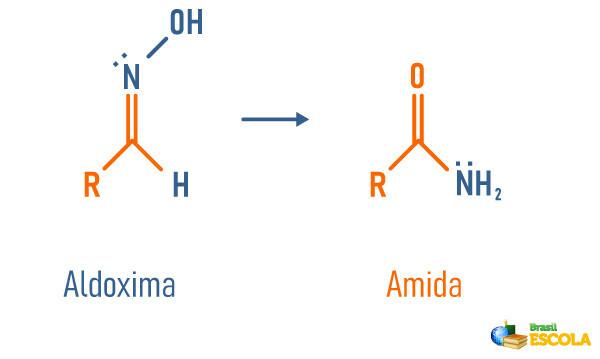
-
Nitrile Hydration
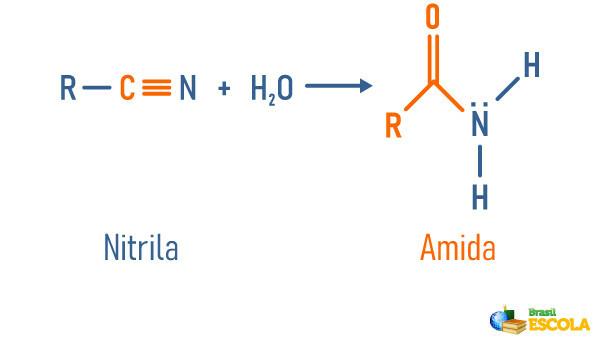
Read too: Amide hydrolysis - reaction used to obtain important substances
solved exercises
Question 1 - (UFRS) Aspartame, pictured below, is an artificial sweetener used in many soft drinks and low-calorie foods.

The group framed in the figure is characteristic of the organic function
A) ester.
B) amide.
C) amino acid.
D) amine.
E) carbohydrate.
Resolution
Alternative B. The functional group selected in the figure is an amide, due to the presence of a carbonyl (C=O) linked directly to nitrogen (N).
Question 2 - (UNESP) In August 2005, the seizure of batches of lidocaine that would have caused the death of several people in Brazil, due to manufacturing problems, was reported. This drug is a local anesthetic widely used in endoscopic exams, reducing patient discomfort. Its molecular structure is shown below:
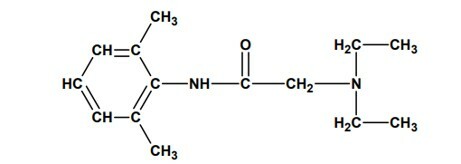
and presents the functions:
A) secondary amine and tertiary amine.
B) amide and tertiary amine.
C) amide and ester.
D) ester and tertiary amine.
E) ester and secondary amine.
Resolution
Alternative B.
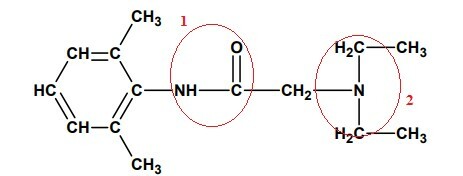
After selecting and numbering the characteristic parts of each organic function, let's analyze each one:
1- It is an AMIDA due to the presence of the acyl group, (R-C=O) directly linked to nitrogen, monosubstituted.
2- As we do not have the presence of the acyl group (R-C=O), but only the nitrogen bonded directly to other carbons in this group we have a TERTIARY AMINE, because all three hydrogens, previously bonded to nitrogen, have been replaced by groups Organic.
By Laysa Bernardes Marques de Araújo
Chemistry teacher
Would you like to reference this text in a school or academic work? Look:
ARAúJO, Laysa Bernardes Marques de. "Amides"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/amidas.htm. Accessed on June 27, 2021.
Amines, classification of amines, amine properties, primary amine, nitrogenous organic compounds, alkyl radicals, dimethylamine, ethylamine, trimethylamine, compounds extracted from vegetables, putrescine, cadaverine, organic bases, syntheses organic