There are three natural radioactive emissions: alpha (α), beta (β) and gamma (γ). Some scientists went on to study the nature of these emissions and some general laws for radioactivity were created. Among these scientists, one who made notable contributions to the study of natural radioactive decay was the English physicist and chemist Frederick Soddy (1877-1956).

A postage stamp printed in Sweden circa 1981 shows an image of 1921 Nobel Prize winner in Chemistry Frederick Soddy*
THE first law of radioactivity, also known as Soddy's first law, it has to do with alpha decay. See what this law says:
“When an atom undergoes alpha decay (α), its atomic number (Z) decreases two units and its mass number (A) decreases four units”.
Generically, we can represent this law by the following equation:
ZTHEX →24α + Z-2A-4Y
This happens with every radioactive element that emits an alpha particle, because as shown in the text Alpha emission (α), this particle is made up of two protons and two neutrons — similar to what happens with the nucleus of a helium atom — and is represented by24α.
The atomic number (Z) is the same as the number of protons. Thus, since with the emission of an alpha particle two protons are lost, the atomic number decreases by two units. The mass number (A) corresponds to the sum of the protons with the neutrons. Since the alpha particle has two protons and two neutrons, the mass number decreases by four units when the nucleus emits such a particle.
Here's an example: Uranium-235, when undergoing alpha decay, results in thorium. Note that your atomic number has decreased by exactly two units (92 – 90 = 2) and your mass number has decreased by four units (235 – 231 = 4):
92235U → 24α + 90231Th
The atomic number and the mass number remain the same in the first and second member of this equation. So, if you want to find out which particle was emitted or which element was originated, just list these quantities.
Looking at the periodic table, we see that thorium is located two places before uranium. This is obvious because the chemical elements are arranged on the periodic table in ascending order of atomic number and the atomic number has decreased by two units:
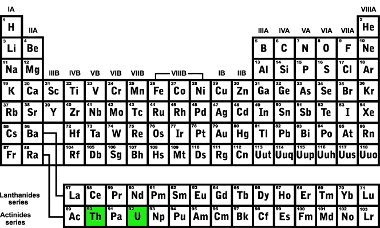
Location of the elements thorium and uranium in the Periodic Table
This brings us to another generalization:
Every atom that emits an alpha particle becomes the atom of the element two places to the left of the original element.
__________________
* Copyrighted image: catwalker / Shutterstock.com.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/primeira-lei-radioatividade-ou-primeira-lei-soddy.htm
