Despite all the achievements of women in the fight against gender inequality, when it comes to sex, there is still a great deal of taboo and prejudice. This statement can be evidenced by the fact that, for many years, no product was manufactured with the purpose of increasing female sexual desire, as if this topic were of interest only to men.
In August 2015, however, the first step was taken, as the first drug with the promise of increasing female sexual desire was approved by Food and Drugs Administration(FDA). With this drug, it is expected that many women have the opportunity to improve their sex life and, consequently, their quality of life.
The medicine called Addyi is composed of the drug flibanserin, which works in the treatment of hypoactive sexual desire disorder in women in pre-menopause. This problem is characterized by the decrease or total disappearance of sexual desires and fantasies.
The new drug that became known as "female viagra" does not work like products suitable for men
, which act only on Organs sexual organs. Flibanserin acts on the central nervous system, more precisely on neurotransmitters related to sexual desire. This medication must be taken daily and does not have immediate effect, taking a few weeks for the benefits to be realized. If no change is observed within eight weeks, it is recommended that the use of the drug be discontinued.Do not stop now... There's more after the advertising ;)
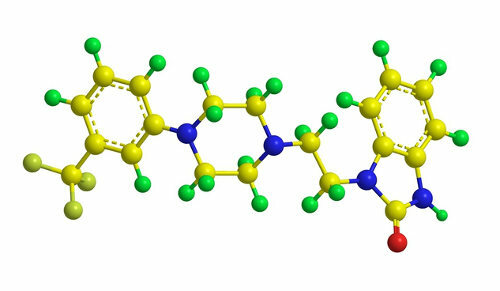
Note the molecular structure of flibanserin, the drug present in female viagra
Some doctors don't believe the drug will solve the female problem, considering sexual desire to be much more complex than the release of neurotransmitters. According to subject matter experts, lack of desire can be caused, for example, by relationship strain, hormonal problems, and even depression. In these aspects, the drug is not efficient.
In addition to these factors, “female viagra” is responsible for triggering some side effects. Among the main adverse reactions of the product are drowsiness, nausea, dizziness, fainting and low blood pressure. These effects are greatest in people who do alcohol consumption. Women with liver failure and who use inhibitors of the enzyme CYP3A4 should not use the drug.
The FDA had previously barred the approval of “female viagra” for commercialization. The agency's claim was that the drug had little efficacy and several side effects. Many doctors claim that the drug's approval in August 2015 was due to pressure from various entities and that Addyi is still unsatisfactory and has several adverse reactions.
By Ma. Vanessa dos Santos
Would you like to reference this text in a school or academic work? Look:
SANTOS, Vanessa Sardinha dos. "“Female Viagra”"; Brazil School. Available in: https://brasilescola.uol.com.br/sexualidade/viagra-feminino.htm. Accessed on June 27, 2021.