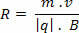THE temperature it is a measure of the degree of vibration of the molecules that make up a body. If the molecular vibration is high, the body will be hot. If the molecular vibration is not intense, the body will be cold.
The exact determination of temperature values in our daily lives is extremely important. As an example, we can mention the determination of body temperature for the diagnosis of fever and the maintenance of exact temperature values for the packaging of medicines.
Bodily sensations cannot be used to exactly set the temperature of a substance, as the human body is not a good thermometer. Thus, temperature can be determined by the behavior of materials against variations of this physical magnitude. We know, for example, that, when undergoing temperature variations, materials can suffer dilation or contraction, thus, it is possible to take advantage of this property to measure its temperature.
You thermometers most common are those of Mercury, in which this liquid metal is stored in a glass bulb, with a certain thermometric scale. Temperature values are marked by the expansion or contraction of this metal.
thermometric scales
The process of building a thermometric scale it's simple and involves just two steps. With a glass bulb where there is mercury, do the following:
1) Marking of fixed water points
Under normal conditions of temperature and pressure, water will always suffer melting and boiling at the same temperatures. Therefore, the bulb with mercury must be joined to a certain amount of ice in the process of melting. When the mercury level inside the bulb has stabilized, the position of the fusion point. Then, joining the glass bulb with boiling water, wait for the stabilization of the mercury level and mark the boiling point.
Whenever the mercury level reaches one of the marked points, we will know that the temperature corresponds to the melting point or boiling point of water.
Do not stop now... There's more after the advertising ;)
2) Assignment of values
After marking the fixed points, values must be assigned to each one of them. Thus, a thermometer will be created on a certain thermometric scale.
thermometric scales
There are currently three thermometric scales in use around the world:
1) Celsius scale:Created in 1742 by the Swedish physicist Anders Celsius (1701 – 1744), this scale assigns the value 0 °C for the melting point and 100 °C for the boiling point of water.
2) Fahrenheit scale:Created in 1708 by the German physicist Daniel Fahrenheit (1686 – 1736), this scale is mainly used in English-speaking countries and has a value of 32°F for the melting point and 212°F for the boiling point of Water.
3) Kelvin Scale: This scale was created by Englishman Willian Thompson (1824 – 1907), known as Lord Kelvin. Referring to the temperature of the absolute zero, temperature at which molecular vibration ceases, the Kelvin scale is known as the absolute scale.
Lord Kelvin assigned a zero value to the temperature of – 273.15 °C, which corresponds to absolute zero temperature. Thus, the melting and boiling points on the Kelvin scale correspond, respectively, to 273 K and 373 K. This scale does not have the degree (°) notation and is used by the scientific community.

Conversion between thermometric scales
The following equation makes the transformation between the temperatures of the Celsius, Fahrenheit and Kelvin scales. By applying it, we can transform any temperature value and find its corresponding one on another thermometric scale.

In this equation, TÇ, TF and TK represent any temperature on the Celsius, Fahrenheit and Kelvin scales, respectively.
Example
Let's use the transformation equation to find the value corresponding to 45 °C on the Fahrenheit scale.

A temperature of 45 °C corresponds to 113 °F.
By Joab Silas
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
JUNIOR, Joab Silas da Silva. "Conversion between thermometric scales"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/conversao-entre-as-escalas.htm. Accessed on June 27, 2021.