THE formation enthalpy, also called standard enthalpy of formation, or standard heat of formation, is the calculation of the heat released or absorbed in the formation of 1 mole of a substance from simple substances, in the standard state.
It is impossible to calculate the absolute value of the enthalpies of each substance, but it is possible to calculate the variation in the enthalpy that occurs in the reaction by means of a calorimeter.
It is necessary to remember that it was agreed to adopt the enthalpy value equal to zero for simple substances in the standard state. Thus, if we want to find out what is the enthalpy of formation of a substance, we only need to know the value of the enthalpy of its formation reaction from simple substances.
For example, we want to find the enthalpy of 18 grams of water, which corresponds to 1 mol, since its molar mass is 18 g/mol. To do this, we first need the reaction to form water from simple substances, as shown below:
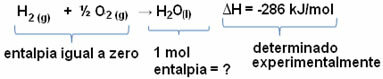
Note that the value of the enthalpy change that occurred in this reaction was experimentally determined by means of a calorimeter and is equal to -286 kJ/mol.
The formula that calculates this enthalpy change (ΔH) is:
ΔH = HProducts - HReagents
Thus, since we already know the value of ΔH and that the enthalpy of the reactants is equal to zero (since they are simple substances in the standard state), we can then conclude that the enthalpy value of 1 mol of water is equal to the enthalpy change of the formation reaction, as it is the only product of that reaction, as shown bellow:
ΔH = HProducts - HReagents
-286 kJ/mol = HH2O - (HH2 + H1/2 O2)
-286 kJ/mol = HH2O - 0
HH2O = - 286 kJ/mol
This type of enthalpy, achieved from the enthalpy of simple substances in the standard state, is therefore the standard enthalpy of formation (ΔH0).
Now, there are many substances that are not formed directly by a single reaction, such as water. In such cases, the enthalpy of formation can be calculated from the enthalpy variation of the reaction.
For example, NH4Cl is formed by the following reaction:
NH3 + HCl → NH4Cl ΔH = -176 kJ/mol
Note that none of the reactants are a simple substance, so we cannot assign them an enthalpy of zero. We need to know the enthalpies of formation of each of the reagents, as these are formed by reactions between simple substances:
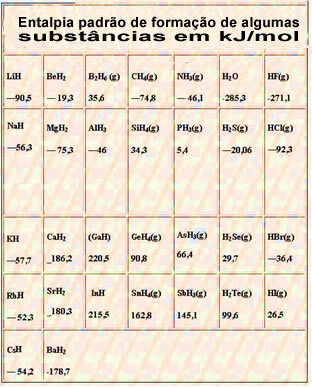
NH3: ΔH = -46 kJ/mol
HCl: ΔH = -92.4 kJ/mol
Adding these two enthalpies, we get the value of the enthalpy of the reactants and we can find the enthalpy of NH4Cl:
HR = HNH3 + HHCl
HR = (-46 + (-92.4) kJ/mol)
HR = -138.4 kJ/mol
Substituting in the formula:
ΔH = HProducts - HReagents
-176 = HNH4Cl - ( -138,4)
HNH4Cl = - 176 - 138,4
HNH4Cl = -314.4 kJ/mol
In this case, we directly summed the values of the enthalpies of formation of the reactants because the reaction ratio was only 1 mol. However, if in other reactions the amount of moles is different, it will be necessary to first multiply the enthalpy of formation of the reactant by the number of moles.
Below is a table with the standard enthalpy of formation of some substances at 25 °C and 1 atm:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/entalpia-formacao.htm
