THE Electrochemistry is a branch of Physical Chemistry that studies the reactions in which there is electron transfer (oxi-reduction reactions) and its conversion into electrical energy, as well as the opposite process, that is, the conversion of electrical energy into energy chemistry.
The first process is what takes place in the Batteries. Batteries are devices formed by two electrodes (a positive pole, which is the cathode, and a negative pole, which is the anode), in addition to an electrolyte (conductive solution). Electrons are transferred by an external conductor from the anode to the cathode, forming an electrical current that is used to turn on some device. Batteries consist of several cells connected in series or in parallel.
This is a spontaneous process and energy is supplied until the chemical reaction is exhausted (as is the case with primary cells and batteries, such as the Leclanché dry cell and the battery alkaline), or, in the case of reversible reactions, a potential difference can be applied and the reaction reversed, forming the reactants again and recharging the battery that is ready to be used again (this is the case of batteries and secondary batteries, such as lead, used in cars, and lithium ion, used in appliances cell phones).

Batteries and primary batteries in the foreground and, in the second, recharge of secondary batteries (lead and lithium ion)
Do not stop now... There's more after the advertising ;)
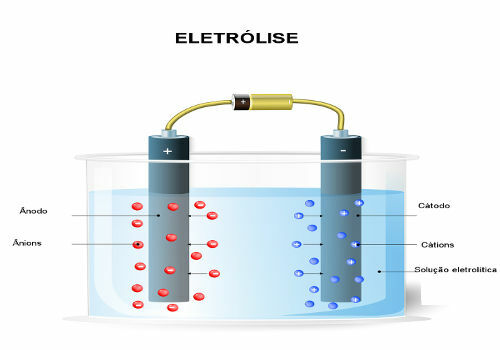
The inverse process, on the other hand, is not spontaneous and is called electrolysis. Electrolysis is the passage of electrical current coming from a generator, such as a cell or battery, through an ionic liquid. If the liquid is some molten substance, we have a igneous electrolysis, but if it's an aqueous solution, we have a aqueous electrolysis.
When passing the electric current over the liquid medium, the generator "pulls" the electrons from the positive pole (anode - it is the opposite of the battery) of the electrolytic cell and transfers them to the negative pole (cathode), that is, the cathode undergoes reduction and the anode undergoes oxidation. Thus, the electrical energy supplied by the generator is transformed into redox reactions (chemical energy). Below is a water electrolysis scheme:
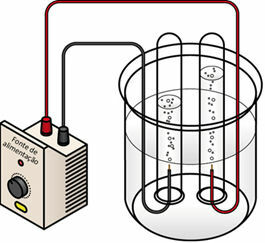
Water electrolysis scheme
Electrolysis is widely applied industrially in the production of important substances, such as aluminum, chlorine gas, metallic sodium, and to purify or protect various metals, as in electroplating or electroplating processes, which consist in the coating of some object by a metal, such as silvering, copper plating, nickel plating, gilding and chromation; when steel is coated with zinc, it is called galvanizing.
In the section of Electrochemistry on our website you will find more details about all aspects related to cells and batteries, as well as electrolysis.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "What is Electrochemistry?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-eletroquimica.htm. Accessed on June 27, 2021.
Chemistry
Electrolysis, electrolyte solutions, electric current, oxidation-reduction reactions, spontaneous chemical process, chemical process non-spontaneous, transformer, artificial transformation, industries, alkali metals, alkaline earth, hydrogen gas, gas cl

