The physical states of matter are determined according to the degree of agitation in which the molecules of this matter are.
In the solid state, the molecules are bonded together due to the very strong cohesion force between them, making the solids present a well-defined volume.
In the liquid state there is a median separation between the molecules, existing between them a non-cohesive force. as intense as in the solid state, making the liquids adhere to the shape of the containers that contains.
In the gaseous state, the molecules are farther apart and the cohesive force between them is relatively small. In this state, matter has no definite volume or shape.
The solid, liquid and gaseous states are the most well-known aggregation states of matter, but others not so well known exist; the Bose-Einstein condensate is one of them.
Bosons (particles that have a spin other than 1/2), when conditioned to a temperature close to zero absolute, reach the lowest quantum state, under these conditions the quantum effects can be visualized in scale macroscopic.
It is a mistake to think that the states of matter are reduced to three, the Bose-Einstein condensate is one of those that are known to few. The lack of knowledge about others is related to the extreme conditions in which the material must be conditioned, thus hindering their dissemination outside the scientific world.
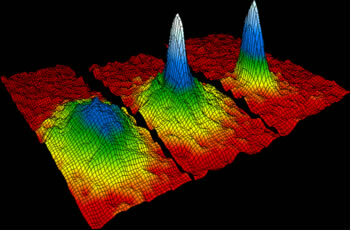
Graph representing the Bose-Einstein Condensate;
a new state of matter.
By Frederico Borges de Almeida
Graduated in Physics
Brazil School Team
Modern physics - Physics - Brazil School
Source: Brazil School - https://brasilescola.uol.com.br/fisica/o-condensado-boseeinstein.htm

