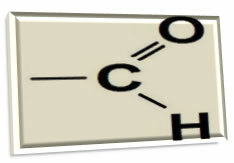
You aldehydes are organic compounds that have as a functional group a carbonyl (carbon atom making a double bond with an oxygen atom) linked to a hydrogen. This functional group (carbonyl + hydrogen) is called aldoxyl, formyl or methaneyl:
Aldehydes are often confused with ketones, as both have the carbonyl group in their structures. However, the biggest difference between these two organic functions is that in aldehydes the carbonyl always comes at the end of a carbon chain, because oxygen is linked to a carbon, which, in turn, is directly linked to a hydrogen; thus, carbon already has three bonds, with only one bond missing to complete four. In the case of ketone, oxygen is attached to a carbon that is between other carbons, therefore, inside the carbon chain and not at the ends.
Because of these points mentioned, the nomenclature of aldehydes is very similar to that of ketones, with only two different points:
Do not stop now... There's more after the advertising ;)
1. O suffix is "al", while in ketones it is “one”;
2. in the aldehydes it is not necessary to locate the position in the carbon chain from which the functional group is leaving, for, as said, he will always come at the end.
Thus, we have as a general scheme of the official nomenclature of aldehydes the following:

Examples:

If there is the presence of unsaturations (double or triple bonds) and branches, it is necessary to number the chain starting with the aldoxyl carbon and locating the unsaturations and branches within the chain, as in the example bellow:

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Aldehyde nomenclature"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/nomenclatura-dos-aldeidos.htm. Accessed on June 27, 2021.