“Chemical bond” was a term first used by Gilbert Newton Lewis in the year 1920 in an article to explain why that atoms stick together to form substances and also why they stick together over thousands of years old.
The atoms of most chemical elements hitherto known and listed in the Periodic Table do not appear in isolated form in nature. Most materials present in our daily lives are substances that can be simple (consisting of atoms of only one type of chemical element) or composites (have atoms of two or more chemical elements many different).
This is because atoms have the ability to make chemical bonds with other atoms, which can be the same element or different elements. These bonds are so strong that without any external influence, in most cases the atoms will remain joined as they are.
Mind Map: Chemical Bonds

*To download the mind map in PDF, Click here!
For example, it is not common to find a free oxygen atom in nature; however, we find several substances in which it appears attached to other atoms. An example of a simple substance is oxygen gas in which each molecule is made up of two bonded oxygen atoms (O
2); while an example of a composite substance is water, where each molecule has two hydrogen atoms bonded to an oxygen atom (H2O).The only elements that are found stably isolated in nature are the noble gases, that is, the elements of family 18 of the Periodic Table (He, Ne, Ar, Kr, Xe and Rn). All these elements have in common the fact that they have eight electrons in the last electron shell (valence layer), with the exception of helium (He), which has only one electron shell (K layer) and therefore holds two electrons, which is the maximum possible number of electrons in that layer.
Thus, Gilbert N. Lewis and also scientist Water Kossel came to the conclusion that the atoms of the other elements bind to have eight electrons (or two, if you have only the K shell) and thus stabilize. It was created, then, the electronic theory of valence, which indicates how many chemical bonds the atom of an element makes, based on the explained idea.
Therefore, atoms make chemical bonds, seeking to lose, gain or share valence shell electrons until they reach the configuration of the next noble gas.This theory also came to be called octet rule.
For example, oxygen is bivalent because it has six electrons in its valence shell. Therefore, it needs to receive two more electrons to have a configuration of the noble gas neon (Ne), that is, with eight electrons in the valence shell, which in this case is the L shell. In the case of the oxygen gas and water mentioned, we have the following:
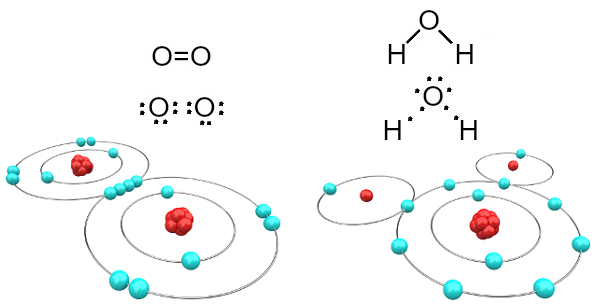
Oxygen and water molecules formed by covalent bonds
Note that in the first case (oxygen gas - O2), each oxygen atom shares two electrons, both of which have eight electrons in the valence shell. This means that a double bond (two bonds at the same time between two atoms).
In the case of water, each of the two hydrogen atoms shares an electron with the central oxygen atom and all are stable (oxygen has eight electrons in the valence shell and each hydrogen has two electrons). Here also two simple connections are made.
This type of chemical bond, in which all atoms need to receive electrons (hydrogen, non-metals and semimetals) and in which electrons are shared in pairs, is called covalent bond.
But there are two more types of chemical bonds:
(1) ionic bond → there is the definitive transfer of electrons from one atom to another. This type of bond occurs between metal atoms (which have a tendency to lose electrons to stay stable) and hydrogen atoms, non-metals and semi-metals (which have a tendency to gain electrons to stay stable).
An example is sodium chloride (NaCl - table salt) where sodium is a metal that has a tendency to lose an electron, while chlorine is a non-metal that has a tendency to gain an electron. Thus, sodium donates(red arrow)an electron to chlorine, forming salt, a very stable substance. Since they graduate (black arrow) ions, which are chemical species with opposite charges (+ and -), one ion attracts another nearby and ionic clusters are formed with a huge number of ions, as are the crystals in table salt.

Formation of sodium chloride through ionic bonding
(2) Metal connection → It is a theory that metals (such as aluminum, gold, silver, copper, etc.) are formed by a cluster of neutron atoms and cations that are held together by a kind of "cloud" of free electrons (electrons that were lost in the formation of cations cited). This cloud (or sea) of electrons would act as a metallic bond that would hold the atoms together.
For more details on these types of chemical bonds, as well as the octet rule, read the related articles below.
Mind Map By M.e Victor Ricardo Ferrreira
Chemistry teacher
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-uma-ligacao-quimica.htm
