O ammonium nitrate is a solid substance of molecular formula NH4AT THE3 and white appearance (when pure). constituted by the ions NH4+ (ammonium) and NO3- (nitrate), is not flammable, but is highly oxidizing and reactive. Its decomposition reaction generates a large volume of gases as the product, which makes inappropriate storage of this product a serious problem, as explosions may occur. And also widely used in agriculture as a fertilizer.
Read too: Natural substances - molecules originated from nature
What is Ammonium Nitrate?

Ammonium Nitrate it's a salt constituted by NH ions4+ (ammonium) and NO3- (nitrate). It's a solid compound that, when pure, it is white in color. It is widely used as an agricultural fertilizer, but it can also be used in the manufacture of explosives, as it's a very oxidizing substance, then oxidizing. The runaway reaction of ammonium nitrate generates a high release of gases, causing explosive impact.
Characteristics of ammonium nitrate
Molar mass: 80.04336 g/mol.
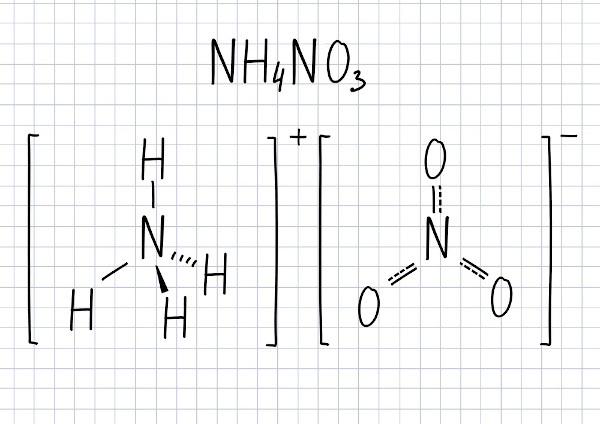
Salt composed of the ions: ammonium (NH4+) and nitrate (NO3-).
Appearance: white solid.
It is strong oxidizing.
Easily reacts with other substances.
It is not flammable.
For normal temperature and pressure conditions:
- density: 1.73 g/cm³;
- boiling point: 210 °C;
- melting point: 169.6 °C;
- degree of solubility in water: 216 g/100ml;
- pH: 5.5 (0.2M).
Ammonium Nitrate Formula
The molecular formula of this compound is NH4AT THE3.
Decomposition of ammonium nitrate
for being a substance with high reactivity and high oxidizing potential, ammonium nitrate, when subjected to high temperature, triggers a decomposition reaction, and the main products are gases, nitrogen, oxygen and water vapor. The decomposition is given in two steps:
→ 1st step:
2NH4AT THE3 (s) → 2N2O(g) + 4H2O(g)
→ 2nd stage:
2N2O → 2N2(g) + O2(g)
→ Global equation:
2NH4AT THE3 (s) → 2N2(g) + O2(g) + 4H2O(g)
Notice that, in the first equation,one of the products obtained in the decomposition is the nitrous oxide (N2O), also known as laughing gas. It is used to increase the power of automotive engines to burn fuel and as an anesthetic, mainly by dentists. It's also one of the main gases responsible for greenhouse effect.
See too: What are the types of chemical reactions?
Ammonium nitrate production
Ammonium nitrate can be obtained by reaction between ammonium sulfate (NH4)2ONLY4 and calcium nitrate Ca (NO3)2. See below:
(NH4)2ONLY4 + Ca (NO3)2 → 2 NH4AT THE3+ Case4
It can also be produced from gaseous ammonia, which is bubbled in a nitric acid solution, having as a reaction product the aqueous ammonium nitrate. Look:
NH3(g) + HNO3(aq) → NH4AT THE3(here)
Uses of Ammonium Nitrate

- Fertilizer Manufacturing
- Explosives in mines, quarries and construction
- Herbicides and Insecticides
- Obtaining nitrous oxide for industrial application
- Absorbents for oxides
- Oxidant for rocket propellants
- Manufacture of isothermal bags
- Production of anesthetic smoke for the management of hives in beekeeping
Also access: How to fight a fire?
Ammonium Nitrate Hazards
One of the main risks associated with ammonium nitrate is in inadequate storage. As it is a very oxidizing substance and it reacts with several other compounds, it triggers a reaction with the release of a large volume of gas. Improper storage is subject to contamination with flammable products, exposure to heat or gas retention, what can cause explosions.
This has already happened in Texas, in the United States, for example, with a cargo ship that came from France carrying 2,086 tons of ammonium nitrate. the accident happened in 1947 and became known as the Texas City Disaster. The tragedy caused the death of 581 people and it occurred due to a fire not contained in the ship that reached the ammonium cargo, triggering the explosion.
Another example was the explosion sequence which happened on August 4, 2020 at the port of city of Beirut, Lebanon, leaving more than 100 people dead and 5,000 injured. The magnitude of the explosion was due to the ignition of a chain reaction of the cargo of 2,750 tons of ammonium nitrate stored there.
It is also worth noting that ammonium nitrate can cause skin and eye irritation and, if inhaled, breathing problems. In the bloodstream, the substance has toxic power, as it reacts with body fluids, and the products of this reaction, such as nitrate, can cause damage to health, such as blue baby disease, also known as infantile methemoglobinemia (low blood hemoglobin). This disease is recurrent in families in rural areas due to contamination with ammonium nitrate, present in fertilizers, of water bodies in the farming region.
by Laysa Bernardes
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/nitrato-de-amonio.htm
