THEdeelhearing is a laboratory, industrial or domestic procedure in which a certain volume of solvent (pure) is added to or removed (through evaporation) from a pre-existing solution.
In any solution, there is always the presence of a solvent and at least one solute, as in a mixture of 500 mL of water and 10 g of sodium chloride (NaCl) represented below:
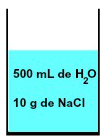
Representation of the mixture formed by water and sodium chloride
If a volume of 300 mL of water is added to this solution, this procedure is called dilution, as the volume of the solution increases – in this case, to 800 mL –, however, without changing the amount of solute.

Dilution by adding solvent to saline solution
THE dilution it can also be performed by heating this saline solution (composed of 500 mL of water and 10 g of NaCl) until, for example, 300 mL of water are vaporized. In this case, the volume of solution would be reduced, however, the amount of solute would not be changed.

Dilution by solvent vaporization in saline solution
Principles of dilution:
The final volume of the solution, when solvent is added, is always greater than the initial volume;
The final volume of the solution, when the solvent is removed, is always smaller than the initial volume;
The mass of the solute never changes when diluting a solution;
The mol number of the solute never changes when diluting a solution;
When solvent is added to a dilution, the concentration of the final solution is always lower than the concentration of the initial solution;
When solvent is removed in a dilution, the concentration of the final solution is always greater than the concentration of the initial solution.
Formulas used in dilution
⇒ Calculation of the final volume of the solution:
The volume of the final solution in a dilution in which solvent is added is calculated by the following expression:
Vf = Vi + VThe
Vf = volume of final solution
Vi = volume of initial solution
VThe= volume of solvent that was added
If there is the removal of solvent in a dilution, the final volume will be calculated by the following expression:
Vf = Vi - Vand
Vand = volume of evaporated solvent.
⇒ Calculation of common concentration:
The concentration of the final solution, after dilution, can be calculated as follows:
Çi.Vi = Cf.Vf
Çi= common concentration of initial solution
Vi = volume of initial solution
Çf = molarity or concentration in mol/L of the final solution
Vf= volume of final solution
⇒ Calculation of molarity or concentration in mol/L:
The molarity of the final solution, after a dilution, can be calculated by the following expression:
Mi.Vi = Mf.Vf
Mi= molarity or concentration in mol/L of the initial solution
Vi = volume of initial solution
Mf = molarity or concentration in mol/L of the final solution
Vf= volume of final solution
⇒ Bulk Title Calculation:
The title of the final solution, after a dilution, can be calculated by the following expression:
Ti.mi = Tf.mf
Ti= title of initial solution
mi = mass of initial solution
Tf = title of final solution
mf= mass of final solution
As the title can also be calculated as a percentage and, in aqueous solutions, the mass tends to have the same value as the volume, we can use the following mathematical expression:
Pi.Vi =Pf.Vf
Do not stop now... There's more after the advertising ;)
Pi= percentage of initial solution
Vi = volume of initial solution
Pf = percentage of final solution
Vf= volume of final solution
Examples of calculations performed in dilution:
1st Example - (UFBA) By adding 300 mL of water to 100 mL of 8% sodium bicarbonate solution, the concentration of the solution obtained is:
a) 24% b) 18% c) 9% d) 4% e) 2%
Data provided by the exercise:
Percentage of initial solution (Pi) = 8%
Initial solution volume (Vi) = 100 mL
Final solution volume (Vf) = 400 mL (result of mixing from 300 mL to 100 mL)
Percentage of final solution (Pf) = ?
To calculate the percentage concentration of the solution, we can use these values given in the following expression:
Pi.Vi =Pf.Vf
8,100 = Pf.400
800 = Pf.400
Pf = 800
400
Pf = 2%
2nd Example - (UFPA) 200 mL of a magnesium hydroxide solution, Mg (OH)2, were prepared by dissolving 2.9 g of the base in water. What volume of this solution must be diluted to 300 mL in order to obtain a solution with a molarity equal to 0.125 M? Data: H = 1; Mg = 24; O = 16.
a) 450 mL b) 150 mL c) 400 mL d) 300 mL e) 900 mL
Data provided by the exercise:
Mass of solute in the initial solution (m1) = 2.9 g
Volume of solution to be used for dilution = 200 mL or 0.2 L (after dividing by 1000)
Initial solution volume (Vi) which will be diluted = ?
Final solution volume (Vf) = 300mL
Molarity or concentration in mol/L of the final solution (Mf) = 0.125M
To calculate the concentration as a percentage of the solution, we must do the following:
Step 1: Calculate the molar mass of the solute.
To do this, we must multiply the number of atoms of each element by its respective atomic mass and then add the results:
MMg(OH)2 = 1.24 + 2.16 + 2.1
MMg(OH)2 = 24 + 32 + 2
MMg(OH)2 = 58 g/mol
Step 2: Calculate the concentration in mol/L or molarity of the initial solution:
Mi = m1
MV
Mi = 2,9
58.0,2
Mi = 2,9
11,6
Mi =0.25 mol/L
Step 3: Determine the volume of the solution that will be diluted using the values provided and found in the following expression:
Mi.Vi = Mf.Vf
0.25.Vi = 0,125.300
0.25.Vi = 37,5
Vi = 37,5
0,25
Vi = 150 ml
3rd Example - (UEG-GO) Consider that 100 mL of an aqueous solution of copper sulfate, with a concentration equal to 40 g. L–1, 400 ml of distilled water was added. In this case, each mL of the new solution will have a mass, in mg, equal to:
a) 2 b) 4 c) 8 d) 10
Data provided by the exercise:
Volume of water added to the dilution = 400 mL
Initial solution volume (Vi) = 100 mL
Final solution volume (Vf) = 500 mL (result of mixing from 400 mL to 100 mL)
Common concentration of initial solution (Ci) = 40 g. L–1
Common concentration of the final solution (Cf) in mg/ml= ?
To calculate the solution concentration in mg/mL, we must do the following:
Step 1: Convert the starting solution concentration from g/L to mg/mL.
To do this, we must multiply both the numerator and the denominator by 1000 and just divide the given concentration by 1000:
Çi = 40g 1000
1L. 1000
Çi = 40 mg/ml
Therefore, the units g/L and mg/mL are the same.
Step 2: Calculate the concentration in mg/mL using the values provided in the following expression:
Çi.Vi = Cf.Vf
40,100 = Cf.500
4000 = Cf.500
Çf = 4000
500
Çf = 8 mg/ml
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is dilution?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-diluicao.htm. Accessed on June 28, 2021.