You polymers they are macromolecules formed by the union of several monomer units (small molecules). One of the classification criteria of polymers is regarding their type of formation, and we have three groups: addition polymers, condensation polymers and rearrangement polymers.
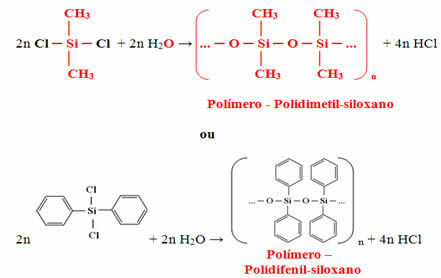
You addition polymers are those that are formed by the sum of successive units of monomers, and generally these monomers are equal and, as a result, are called homopolymers.
There are, however, addition polymers that are formed from different monomers. these are the copolymers. The formation of copolymers can take place in a regular or irregular way. Below we can see that the different monomers can arrange themselves in a regular way, intercalated or in blocks, in a way random, arranging itself at random, and it can also happen that blocks of monomers are grafted as chains sides. These changes modify the properties of the final polymer.

In the above generic examples, two different monomers were used to form the copolymers, however, they can be more than two monomers.
Synthetic rubbers are copolymers, the main ones being three:
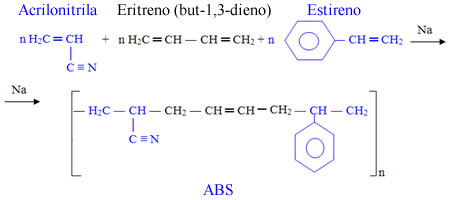
- ABS: This acronym comes from the fact that this polymer is formed by the union of three monomers: acrylonitrile (A), but-1,3-diene (B) and styrene (S). styrene).
Do not stop now... There's more after the advertising ;)
Its main use is in the manufacture of tires, but it is also used in the production of telephones, electrical appliance casings and packaging.
- Buna-N: It is formed by erythrene (but-1,3-diene), where the prefix “bu” comes from, and by acrylonitrile, where a nitrile group comes from, and hence the “N” at the end. The "na" comes from sodium (Na - from the Latin attrium), which acts as a catalyst in the polymerization reaction of this copolymer:
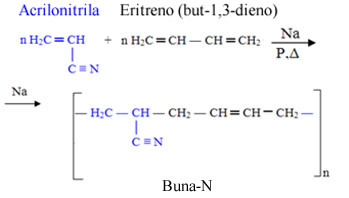
Buna-N is used in hoses, gas tank linings and gaskets.
- Buna-S: It is formed by erythrene (but-1,3-diene) and by styrene (vinylbenzene), which, in English, is written styrenehence the “S” at the end. The “na” also comes from the action of sodium as a catalyst.
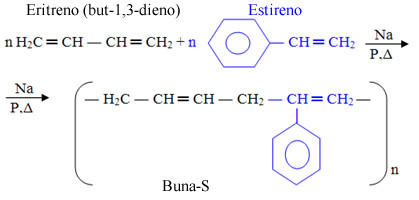
Buna-S is used in electrical cable insulation, tire treads, soles and miscellaneous artifacts.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Copolymers"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/copolimeros.htm. Accessed on June 28, 2021.