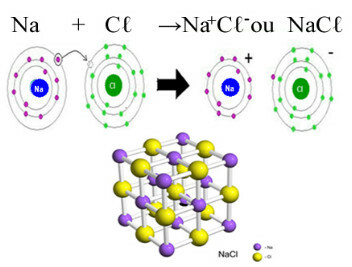
Atoms are infinitely small particles that make up all the matter in the universe. Over time, the idea of what the atomic structure would be like changed according to new discoveries made by scientists. You can find out more about this in the textEvolution of the Atomic Model.
A model is a representation of reality (not reality itself), so atomic models are representations of main components of the atom and its structure and explain certain physical and chemical behaviors of matter. This is done because it is not yet possible for a human being to see an isolated atom even with ultramicroscopes.
To get an idea of how small the atom is, know that The The smallest particle visible under an ordinary microscope contains over ten billion atoms! The atom is so small that, if we put a million of them side by side, we wouldn't reach the thickness of a hair.
Mind Map: Atom
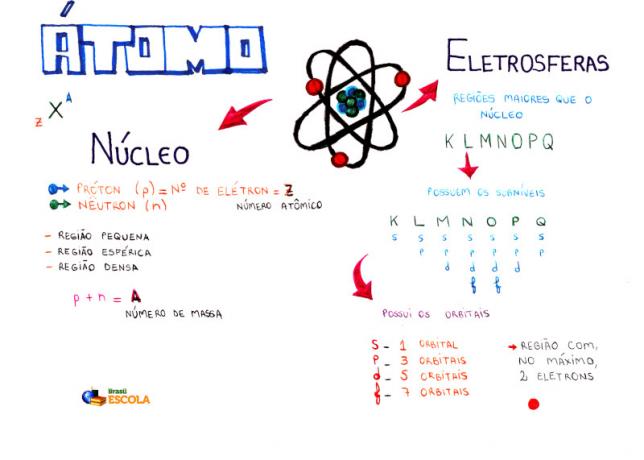
* To download the mind map in PDF, Click here!
Among the atomic models, the most used currently in high school for understanding the structure of the atom and its properties is the Rutherford-Bohr model. According to this model, the structure of the atom consists of two main parts: the
core and the electrosphere.
Two main parts of the structure of an atom - electrosphere and nucleus
* Core: Constituting as the central part of the atom, it is compact, massive and very dense, in addition to being formed by particles of greater mass, which are protons and neutrons.

Illustration of protons and neutrons that make up the atomic nucleus
- Protons: they are particles with a positive electric charge (relative charge = +1; charge in coulomb (C) = +1.602. 10-19) and its relative mass is equal to 1.
The fact that protons form the nucleus and give it an overall positive charge was discovered by Eugen Goldstein, in 1886, through a modification of Crookes' ampoule and some experiments. He saw that, under very high voltages, emissions appeared (anode rays - remnants of atoms of the gas that were inside the bulb and that had their electrons ripped off by the electric discharge). By placing an electric or magnetic field outside the bulb, these rays were deflected towards the negative pole. This meant that there were positive subatomic particles, which were called protons.
Later, Ernest Rutherford (1871-1937) performed the experiment described in the text Rutherford's Atom, which led him to discover the location of the proton: in the nucleus.
- Neutrons: they are particles with a mass equal to that of protons (1), but as the name implies, they are neutral, that is, they do not have an electric charge.
Neutrons were discovered in 1932 by James Chadwick (1891-1974), who realized that the nucleus of radioactive beryllium emitted neutral particles with a mass roughly equal to the mass of protons (actually, it is slightly larger).
Do not stop now... There's more after the advertising ;)
The diameter of the nucleus depends on the amount of protons and neutrons that the atom has, but, on average, it is around 10-14 month 10-15 m.
The atomic nucleus concentrates practically the entire mass of the atom, being a very small part: both the proton and the neutron are about 100,000 times smaller than the entire atom itself! By way of comparison, imagine that we enlarge the nucleus of the atom of the element hydrogen (which has only a proton) to the size of a tennis ball, the closest electron would be about three kilometers from distance! Even if an atom were enlarged to the height of a 14-story building, its core would be the size of a mere grain of salt on the seventh floor. It's really something amazing, don't you think?!
* Electrosphere: Is region where electrons are rotating around the nucleus. Despite being a region of much larger volume than the nucleus, it is practically empty, as each electron is 1836 times smaller than 1 proton (or than 1 neutron). That's why the atom's mass is practically all in the nucleus. Electrons are particles with a negative electrical charge (-1).
Electrons were discovered in 1897 by Joseph John Thomson (1856-1940), the creator of the Thomson atomic model. Thomson's experiment can be seen in detail in the text Thomson's experiment with electrical discharges, but in short, he used the aforementioned Crookes ampoule and realized that cathode rays were always attracted by the positive pole, which proved that the atom had negative particles, which were called electrons.
Electrons spin around the nucleus billions of times per millionth of a second, shaping the atom and making it behave as if it were solid.
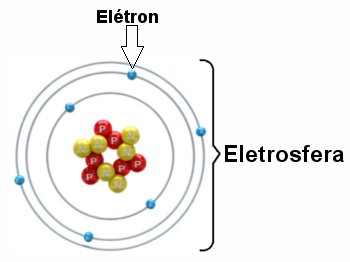
Illustration of an electrosphere with three electronic layers and electrons rotating around the nucleus.
Briefly, we can make a table to differentiate the three main subatomic particles that are part of the structure of the atom:

Mass and electrical charge of the three main subatomic particles - protons, neutrons and electrons
The atoms of all chemical elements are composed of these three subatomic particles. What differs one chemical element from another is the amount in which these particles appear, especially the amount of protons in the nucleus, which is called the atomic number. Keep studying about it through the text. Chemical element.
* Mind Map by Me. Diogo Lopes
By Jennifer Fogaça
Graduated in Chemistry
Chemistry
Classification of matter, water, hydrocyanic gas, carbon dioxide, ammonia, hydrogen, helium, substances simple, compound substances, mixtures, phases of a mixture, homogeneous mixture, mixture heterogeneous.