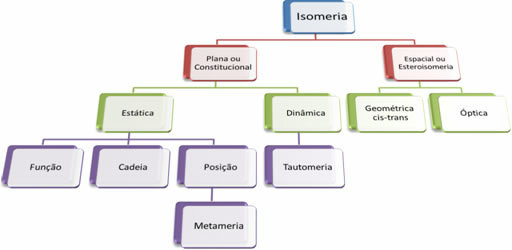
Since there are thousands of organic compounds, the phenomenon of isomerism can take several forms. Therefore, isomerism can be basically classified into two types: flat or constitutional isomerism and space isomerism or stereoisomerism. Each type mentioned can be subdivided, as shown in the following diagram:
See each case:
1. Flat or Constitutional Isomerism: Isomers of this type have the same molecular formula and are distinguished by flat structural formulas. There are five cases of plane isomerism: function, chain, position, metamerism and tautomerism.
1.1.Functional or functional isomerism: The difference between the isomers is in the functional group.
Example: Molecular formula C3H6O
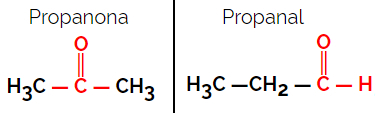
Note that propanone is from the ketone group and propanal is from the aldehyde group.
1.2. Chain or skeletal isomers: The difference between the isomers is in the type of chain. For example, one isomer is open-chain and the other closed-chain, or one is normal-chain and the other branched-chain, or one is a homogeneous chain and the other is a heterogeneous chain.
Example: Molecular formula C4H10
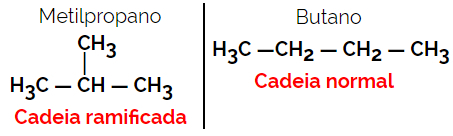
1.3.Positional or Positional Isomerism: The difference is in the position of an unsaturation, a functional group, a heteroatom or a substituent.
Example: Molecular formula C4H6

1.4.Compensation Isomerism or Metamery: It is a special type of position isomerism, where the difference consists of the position of the heteroatom.
Do not stop now... There's more after the advertising ;)
Example: Molecular formula C4H10O
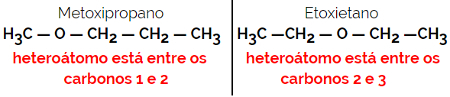
1.5.Dynamic Isomery or Tautomery: It is a special type of function isomerism, in which the isomers coexist in dynamic equilibrium in solution. The two main types of tautomeria are between a ketone and an enol (ketoenol balance) and between an aldehyde and an enol (aldoenol balance).
Example: Molecular formula C3H6O
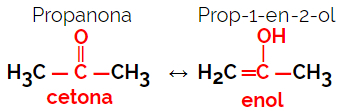
2. Space Isomerism or Steroisomerism: In this case, the difference between the isomers can only be visualized through the orientation of their atoms in space. There are two types of stereoisomerism: geometric isomerism and optical isomerism.
2.1.Geometric or cis-trans isomers: The difference is that the isomer named as cis it has the same carbon ligands in a double bond or in cyclic compounds on the same side of the plane. The isomer ligands trans are on opposite sides.
Example: Molecular formula C2H2Cl2

These compounds are called stereoisomers.
2.2.Optical isomer: Occurs when isomers are able to deflect a polarized light beam. If it bends the polarized light beam to the left, it is a levorotary isomer, but if it bends to the right it is called a right-handed isomer.
Example:
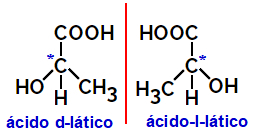
Asymmetric molecules like the ones shown above, which are mirror images of each other and which are not superimposable, are called enantiomers.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Types of isomerism"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/tipos-isomeria.htm. Accessed on June 28, 2021.
Of function, of position, of function, of chain, tautomery, of chain, of chain, metamery.
From position, from function, from chain, from chain, tautomery, from chain, from position, metameria.
From position, from chain, from chain, from chain, tautomery, from chain, from position, tautomery.
From position, from function, from chain, from function, tautomery, from chain, from position, metamery.