O sodium chloride (table salt) is the salt we use in our daily lives to salt handcrafted or processed (industrialized) foods. It is a substance that is also present in various natural foods consumed by us on a daily basis, such as fruits, vegetables, legumes, seeds, etc.
In this text you will know everything about this important substance for the human being's daily life:
the definition
Sodium chloride belongs to the inorganic function of salts and is composed of the association of the sodium cation (Na+) it's the anion cthereoreto (Cl-) through a ionic bond.
b) Chemical characteristics
Sodium chloride is formed by two chemical elements:
→ Sodium (Na):
belongs to the family of metals (capable of forming cations easily) alkaline (AI);
has an electron in the valence shell;
has atomic number equal to 11;
has high electropositivity (ability to lose electrons).
→ Chlorine (Cl)
belongs to the family of halogens (VIIA);
it's a nonmetal (that's why it becomes an anion so easily);
has seven electrons in the valence shell;
has atomic number equal to 17;
has high electronegativity (ability to gain electrons).
As the two chemical elements that form sodium chloride present, respectively, high electropositivity and high electronegativity, between them there is an ionic bond (established between atoms with a tendency to lose and gain electrons).
The chemical structure of sodium chloride is composed of a single chloride anion (green sphere), which interacts with six sodium cations (blue spheres), as can be seen in the structure below:
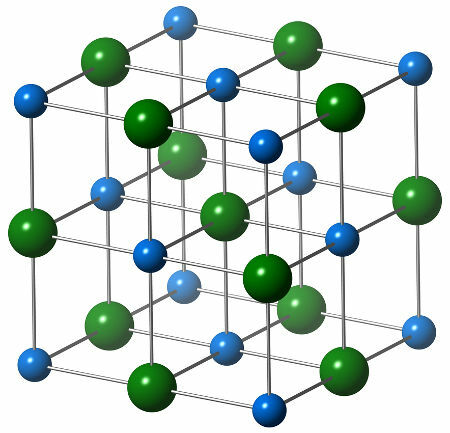
Representation of the crystal structure of sodium chloride
c) Physical characteristics
Fusion point:
Sodium chloride can be transformed from solid state to liquid state at a temperature of 801 OÇ.
Boiling point:
Sodium chloride can be transformed from a liquid to a gaseous state at a temperature of 1465 OÇ.
Polarity
As it is a substance originated by an ionic bond, that is, because it is an ionic compound, sodium chloride is polar.
Solubility in water
We can dissolve in 1 L of water, at 25 OC, up to 359 grams of sodium chloride.
Solubility in other solvents:
As sodium chloride is a polar compound, it cannot be dissolved in any solvent of a non-polar nature, such as oil.
Density:
The density of sodium chloride is 2.165 g/mL, therefore, it is denser than water, which has a density equal to 1 g/mL.
Electrical conductivity:
As it is an ionic compound, sodium chloride is capable of conducting an electric current only when:
-
It is in its molten state, that is, liquid;
Do not stop now... There's more after the advertising ;)
Dissolved in water.
d) Methods of obtaining
Sodium chloride can be obtained physically or chemically:
1O) Getting physical:
fractional crystallization
Sodium chloride is obtained by evaporating water from oceans.
underground mines
It is extracted in mines using mining techniques.
underground deposits
It is extracted from deep underground deposits through dissolution in water (the salt present in the deposit is dissolved) and subsequent pumping.
2O) Obtaining chemically
synthesis reaction
Sodium chloride can be obtained from the chemical reaction of synthesis (simple substances give rise to a compound substance) between chlorine gas and metallic sodium:
2 In(s) + Cl2(g) → 2 NaCl(s)
Neutralization reaction:
Another way to obtain sodium chloride chemically is through the neutralization reaction between hydrochloric acid and sodium hydroxide, in which we have the formation of salt and water:
HCl(1) + NaOH(here) → NaCl(here) + H2O(1)
e) Importance for human beings
Sodium chloride by itself has no function in the human body, but when it dissociates into sodium cations (Na+) and chloride anions (Cl-), each of these two ions has several important functions for our body. See some of these functions:
→ Functions of the sodium cation (Na+)
Prevents blood clotting;
Fights the formation of kidney and gallstones;
Participates in the regulation of body fluids;
Participates in the regulation of blood pressure.
→ Functions of the chloride anion (Cl-)
Participation in the formation and constitution of gastric juice (hydrochloric acid – HCl);
Participation in the formation of pancreatic juice.
f) Damage to the human body
Excessive consumption of sodium chloride can cause the following harm to human beings:
→ Damage caused by excess sodium cations in the body:
Increased wound healing time;
Increased incidence of cramps;
Increased blood pressure;
Kidney overload;
Increased fluid retention in the body.
→ Damage caused by excess chloride anions in the body:
destruction of vitamin E;
Decrease in iodine production in the body.
g) Other applications
In addition to being used to salt food, sodium chloride can also be used in the following situations:
Shampoo production;
Paper production;
Production of Sodium hydroxide (NaOH);
Detergent production;
Soap production;
Snow melting in places that suffer from blizzards;
Production of metallic sodium;
Chlorine gas production;
In isotonics for body electrolyte replacement;
In nasal decongestant solutions;
Production of saline solution; among other applications.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Sodium chloride (table salt)"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/cloreto-sodio.htm. Accessed on June 27, 2021.
Ionic compounds, main characteristics of ionic compounds, bonding between ions, definitive transfer of electrons, electrostatic attraction forces between ions, negative and positive ions, anions, cations, ionic bonding, molecular structure he
Chemistry
Ionic bond, arrangements between ionic compounds, ionic agglomerates, sodium chloride, table salt, ionic substance, electrostatic attraction forces, chloride anions, sodium cations, polar solvents, positive ions, cations, negative ions, anions.


