John Dalton was born on September 6, 1766, in the remote village of Eaglesfield, on the edge of the Lake District in England. His father was a weaver of Quaker belief (in Brazil, it is called Quaker), a term used to identify the members of the Society of Friends, founded in England in the 12th century. Dalton studied at a Quaker community school until the age of 11, and at the incredible age of 12 he began teaching mathematics at that same institution. As his salary was symbolic and he came from a poor family, he also had to work in his father's garden.
John Dalton was a great student of the constitution of matter. For example, he studied different chemical reactions, measuring the masses of reactants before and after the reactions. Thus, in 1808, after much research and scientific experiments, Dalton published a book on the theme New Philosophical System of Chemistry, in which he presented the theory that matter is made up of atoms, which were, according to him, tiny indivisible and indestructible particles.
In addition, he also said that each chemical element was made up of identical and unique atoms, that atoms were immutable, that chemical elements could combine to form different compounds and that chemical reactions were rearrangements of atoms into different compounds, but did not change the total number of atoms that took part in the reaction. He even created a new notation to represent chemical reactions that combined different atoms, as shown in the figure below:
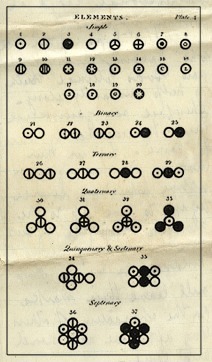
His work was widely debated by the scientific community and famous physicists at the time criticized him. But from the second half of the nineteenth century, chemists began to convince themselves, by the numerous evidence, that such a model was quite plausible. So, dUnlike many scientists who have had to wait many years to get their ideas accepted, Dalton has watched the scientific community embrace his theories. These theories explained many laws that had already been observed (such as Proust's law of constant or multiple proportions).
Do not stop now... There's more after the advertising ;)
Today we know that not everything proposed by Dalton was true, for example: atoms are not indivisible; but his sensational idea transformed our understanding of matter, and his basic premises remain fundamental to our current understanding of chemistry and physics.
John Dalton was also the scientist who introduced the concept of atomic mass, discovered an important law of physics, which is the law of pressure. partial gases, he served as a meteorologist and kept meteorological records for nearly 60 years, having made his last notes on the day of his death.
Another important facet of Dalton's life is that he was the first scientist to describe it thoroughly and scientifically, in 1794, in his book Extraordinary facts relating to the vision ofColors, a visual impairment that he himself had, which became known as color blindness, in his honor. People with this disease cannot distinguish some colors, including red and green.
A basic test for a person to know if they are color blind is to look at the illustrations below. If a person can distinguish the numbers that appear in the illustrations, he is not colorblind.

John Dalton died in 1844, at the age of 78.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "John Dalton"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/john-dalton.htm. Accessed on June 27, 2021.