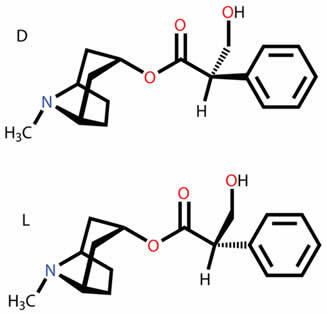
Optical isomerism is linked to the fact that the organic molecule is asymmetric. Most of the time, the asymmetry of the molecule is identified through the presence of a asymmetric or chiral carbon, that is, carbons that have the four ligands different from each other.
However, there are molecules that do not have asymmetric carbons, but that are still asymmetric and, consequently, have optical activity. Among them we have the allenic compounds, that is, derivatives of the allene, the simplest alkadiene that exists:

As can be seen in the case of the generic allenic compound below, although none of its carbons have four different ligands, not being asymmetric, the molecule has a spatial conformation that makes it asymmetric. Furthermore, it is not superimposable in relation to its mirror image:

So, we have two optically active compounds, the right-handed (shifts the polarized light plane to the right) and the levogyro (shifts the polarized light plane to the left). We also have an optically inactive compound, which is the
racemic mixture, that is, 50% right-handed and 50% left-handed. Since each of these isomers deflects the plane of polarized light at the same angle but in opposite directions, one cancels out the other and the mixture has no optical activity.Do not stop now... There's more after the advertising ;)
This can occur with alkadienes with conjugated or consecutive double bonds, as long as they have two different substituents on the two atoms at the ends of the double bonds.
If we are not careful, we can be fooled into thinking that the molecule is symmetric, since it has the same ligands on each carbon atom as on the other carbon. But, as shown below, the molecule is asymmetric:

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Asymmetric carbonless optical isomers"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/isomeria-optica-sem-carbono-assimetrico.htm. Accessed on June 28, 2021.
Chemistry
Know what the various types of plane and spatial isomers are all about, such as function, position, chain, tautomerism, metamerism, cis-trans geometric and optical isomerism.