Over millions of years, wood undergoes a fossilization process, in which the concentration of carbon increases and mineral coal, also called natural coal, is produced.
There are four main types of mineral coals: peat, lignite, hard coal and anthracite. Hard coal is also called stone coal and has about 80% carbon by mass. This variety of charcoal is of great commercial importance, as its dry distillation produces three fractions that are used for the most diverse purposes.
This dry distillation, in the absence of air, occurs by heating the coal to very high temperatures, approximately 1100°C, and the three fractions obtained are: gas fraction, liquid fraction and solid fraction. See the constitution and applications of the components of these fractions:
1. gas fraction: consists of several gases, the main one being hydrogen (H2), followed by methane (CH4), carbon dioxide (CO2), carbon monoxide (CO), nitrogen (N2), olefins and other gases in small quantities.
This fraction is often called lighting gas, as it was often used in gas lamps, in street lighting. Currently, it can be used as fuel in homes and industries.

The gaseous fraction of coal is the lighting gas
2. net fraction: it can be divided into two main parts: ammonia water and coal tar.
2.1. Ammonia waters: are made up of amines, compounds derived from ammonia (NH3) by replacing one of its hydrogens by other organic substituents. It also has ammonium hydroxide base (NH4OH) and ammonium salts.
The main application of ammoniacal waters is in the production of nitrogen fertilizers and agricultural fertilizers.

Ammonia waters are used in the production of nitrogen fertilizers.
2.2. Coal tar: details about the constitution of this coal derivative can be found in the text Tar, but, briefly, we can say that it is a black, thick and strong-smelling liquid, which is constituted by a complex mixture of aromatic hydrocarbons, that is, compounds that are formed only by carbon and hydrogen atoms, having at least one benzene ring as the shown below:
Do not stop now... There's more after the advertising ;)
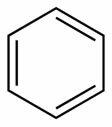
Structure of a benzene ring
Coal tar can also undergo distillation and give rise to five fractions, four of which, the light oil, O medium oil, O heavy oil it's the anthracene oil, they are used for a variety of purposes, such as in the manufacture of plastics, paints, cleaning products and medicines. The components of these fractions, such as benzene, can be separated and used in laboratories for specific purposes. The fifth fraction obtained through the distillation of coal tar is the tar, which is widely used in asphalt paving.

The pitch used in asphalt paving is obtained from coal tar
- 3. solid fraction: it's the coking coal, an amorphous solid, consistent and porous. Its main application is in the steel industry, in the production of iron and steel (metal alloy formed by iron (Fe) in a proportion of approximately 98.5%, carbon (C), with about 0.5 to 1.7%, and traces of silicon (Si), sulfur (S) and oxygen (O)).

Steel is made using coking coal
Thus, the products derived from hard coal are represented in the summary below:
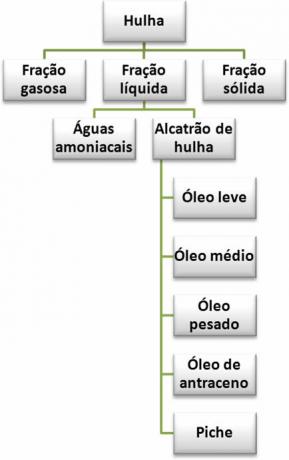
Products from the dry distillation of coal
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Coal"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/hulha.htm. Accessed on June 28, 2021.


