Ionic bonding occurs between ions, as the name suggests. Because they have opposite charges, the cations (element with a positive charge) and anions (element with a negative charge) attract each other electrostatically, forming the bond. However, an ionic solid is constituted by an agglomerate of cations and anions organized with well-defined geometric shapes, called lattices or crystalline lattices.
For example, salt (sodium chloride) is formed by the definitive transfer of an electron from sodium to chlorine, giving rise to the sodium cation (Na+) and the chloride anion (Cl-). In practice, this reaction involves not just two atoms, but an enormous and indeterminate number of atoms that form a cubic-shaped crystalline lattice, as shown below:
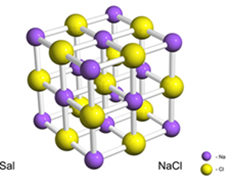
If we look at salt crystals with a scanning electron microscope, we will see that they are actually cubic because of their internal structure.

Since every ionic compound is then made up of an indeterminate and very large number of ions, how can we represent an ionic compound?
The formula usually used is the unit formula, which is the one that represents the proportion expressed by the smallest possible number of cations and anions that make up the crystalline lattice, so that the total charge of the compound is neutralized. For this to occur it is necessary that the number of electrons given up by an atom is equal to the number of electrons received by the other atom.
Some aspects about the unit formula of ionic compounds are important, see some:
Do not stop now... There's more after the advertising ;)
- Always write the cation first and then the anion;
- Since every ionic compound is electrically neutral, the individual ion charges do not need to be written down;
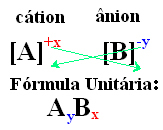
- The subscript numbers appearing on the right side of each ion indicate the ratio of the atoms of the cation to the anion. These numbers are called indices and the number 1 is not written.
For example, in the case of sodium chloride, we have that its unit formula is NaCl, as we have exactly 1 sodium cation for each chloride anion.
See another example, Al3+ has three positive charges, while the F- has only one negative, so three fluoride anions are needed to neutralize the compound. Thus, we conclude that its unit formula is AlF3.
A simple way to arrive at the unit formula of the ionic compound is to exchange its charges for its indices, as shown in a generic way below:
Examples:

Another formula used to represent ionic substances is the Lewis formula or electronic formula, what represents the electrons from the valence shell of the "balls" ions around the element symbol. In the case of salt, we have:

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Formulas for representing Ionic Bonds"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/formulas-para-representar-as-ligacoes-ionicas.htm. Accessed on June 28, 2021.
b) Determine the value of x in the formula for sodium aluminum fluoride.
Ionic compounds, main characteristics of ionic compounds, bonding between ions, definitive transfer of electrons, electrostatic attraction forces between ions, negative and positive ions, anions, cations, ionic bonding, molecular structure he
Carbon spatial formula, Lewis Electronic Formula, plane structure, electronic pairs, bond covalent, valence layer, evolution of the atomic model, molecular formula, structural formula, formulas three-dimensional.
Chemical formulas, flat structural formula, Couper structural formula, triple bond, gas nitrogen, electronic formula, Lewis formula, molecular formula, single bond, double bond, gas carbonic.
Chemistry
Ionic bond, arrangements between ionic compounds, ionic agglomerates, sodium chloride, table salt, ionic substance, electrostatic attraction forces, chloride anions, sodium cations, polar solvents, positive ions, cations, negative ions, anions.