Below are some of the basic concepts that we need to know when starting studies in General chemistry. Observe them:
→ Chemistry
It is science that studies matter, its transformations and the energies involved in these processes. It works at three main levels:
- Microscopic:
When Chemistry interprets phenomena in which there is a reordering of atoms, which are the basic constituents of all matter and which are invisible to our eyes.
- Macroscopic:
When chemistry interprets large, visible objects or phenomena.
- Symbolic:
When Chemistry interprets and recognizes chemical phenomena through symbols, formulas and mathematical equations.
* Depending on the fields of study, Chemistry can be divided into: Organic chemistry, Inorganic chemistry, Physicochemical, between others.
→ Subject
Despite not being such an easy concept to be defined, most authors agree that matter in the study of Chemistry is everything that occupies space in space, presenting volume and mass.
Example: a tree, air, water, clouds, our own body, the earth, all these are examples of matter. But justice, for example, is not.
→ Pasta
It is a general property of matter that indicates the amount of matter that exists in a body and whose standard unit is the kilogram. To measure this property, scales are used.
→ Volume
It is also a general property of matter that indicates the extent of space occupied by a body, its default unit being the cubic meter (m3). The volume of a material can be measured using different devices that are graduated, such as a beaker, a pipette, a burette and other less accurate ones.
→Body
It's a limited portion of the matter. For example, as stated, a tree is a matter; thus, when we cut wood logs, we have that these logs can be designated as bodies or as matter as well.
→Object
It is a body produced for man's use. If the wooden logs mentioned in the previous item are transformed into some furniture, such as a table, we will have an object.
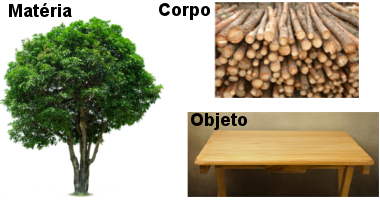
Examples of Chemistry concepts - matter, body and object
→ Energy
It is the measure of the ability to do a job. There are different types of energy, depending on the type of work performed. For example, the energy that a body acquires when it is in motion is the andkinetic energy. The energy that the body stores is the potential energy. THE mechanical energy it is any form of energy related to the movement of bodies or the ability to set them in motion or deform them. THE chemical energy it is based on the force of attraction and repulsion in chemical bonds, present in the formation of matter. Heat exchanges are thermal energies. Conducting electricity is a electricity, and the energy in the form of light is the light energy.
Do not stop now... There's more after the advertising ;)
→ pure substance or simply substance
Substances are materials that have all the physical properties well defined, determined and practically constant, that is, they are formed by a single type of component (atoms, molecules or ionic clusters).
- simple substance:
It is the substance formed by a single type of chemical element. Examples: oxygen gas (O2), hydrogen gas (H2), iron (Fe), helium gas (He), aluminum (Aℓ) etc.
- compound substance or composite:
It is the substance formed by more than one chemical element. Examples: water (H2O), ethyl alcohol or ethanol (C2H5OH), ammonia (NH3) etc.
→ Mixtures
When we have more than one substance in the same system. Mixtures do not have the properties, such as melting and boiling points, as well as density, which are constant as with substances.
- Homogeneous mixture:
It is the mixture that has a single phase, that is, a totally uniform appearance. Example: Mixture of water and alcohol.
- Heterogeneous mixture:
It is the mixture that has more than one phase. Example: Water and oil.
→ System
It is what is being subjected to observation. Regions around the system are called neighborhoods.

System is what is under observation
- Homogeneous system:
Features a single phase. It can be composed of a pure substance or a homogeneous mixture.
- Heterogeneous system:
Features more than one phase. It can be composed of a pure substance in different physical states, such as a glass of water and ice, or a heterogeneous mixture.
→ Phenomenon
Any transformation undergone by matter.
- physical phenomena:
They are those in which the material's constitution does not change. Example: Crumple a paper.
- chemical phenomena:
They are those in which the material's constitution changes. Example: Burning a paper.
More details about these concepts and others, such as atoms, chemical elements and molecules, can be seen in other texts on our website.
By Jennifer Fogaça
Graduated in Chemistry
Chemistry
Molecular substances, boiling temperature, contact surface, boiling point, intermolecular forces of attraction, chemical bond, molecular compounds, covalent chemical bonds, ionic bonds, metallic bonds, physical states of bad