In the text "Igneous Electrolysis”, it was explained that this process occurs when an electric current is passed in a molten substance (in the liquid state), without the presence of water and, in this way, the cation receives electrons and the anion donates electrons, so that both have an electric charge equal to zero and energy accumulated.
To better understand how igneous electrolysis occurs, let's consider one of the most important examples of this type of process, the electrolysis of sodium chloride or table salt (NaCl).
Sodium chloride is formed in nature through the transfer of an electron from sodium (Na) to chlorine (Cl), as per the reaction below:
2Na(s) + 1Cl2(g) → 2NaCl (s)

This process is spontaneous, but the inverse process of this reaction is not spontaneous, that is, the production of chlorine gas (Cl2(g) – figure below) and metallic sodium (Na (s)) does not occur in nature. If we want this to happen, we will have to start the process.
This can be done by igneous electrolysis. The salt is heated to a temperature above 800.4°C, which is its melting point; and in this way it merges, passing from solid to liquid. In this physical state, your Na ions
+ and Cl- are free.The molten salt is then placed in a container, the electrolytic vessel, and two inert platinum or graphite electrodes are dipped in the sodium chloride. These electrodes are connected to a source that generates direct electrical current, such as a battery or a cell.
With the passage of electric current, the following happens:
- The negative pole of the battery or cell supplies electrons to one of the electrodes, which becomes the cathode;
- Cathode: receives the electrons from the cell and becomes the negative pole, attracting the Na cations+, because opposite charges attract. These ions receive the electrons from the electrode (cathode) and their reduction occurs, forming metallic sodium:
Reduction:At+(ℓ) + and- → In(s)
Metallic sodium is deposited on top of the electrode and is sent to a reservoir.
Do not stop now... There's more after the advertising ;)
- Anode: becomes positively charged, attracting Cl anions- (that's why it's called an anode). These ions lose their electrons when they come into contact with the anode and, therefore, they undergo oxidation, forming chlorine atoms, which immediately combine two by two to form chlorine gas:
Oxidation:2Cl-(ℓ) → 2 and- + 1Cl2(g)
This gas is bubbling around the anode and is collected by a glass tube adapted to the system.
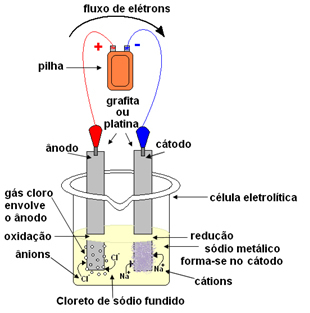
Thus, the overall reaction that occurs in this case is given by:
Cathode: 2Na+(ℓ) + 2e- → 2Na(s)
Anode: 2Cl-(ℓ) → 2 and- + 1Cl2(g) ____________
Global Reaction: 2Na+(ℓ) + 2Cl-(ℓ) → 2Na(s) + 1Cl2(g)
Another important aspect to be aware of, which was highlighted at the end of the mentioned text (Igneous Electrolysis), is that, for electrolysis occurs, the cell or battery used to generate the electrical current must have a ddp (potential difference) equal to or greater than the potential difference of the reaction.
Let's look at this in the case of sodium chloride electrolysis we are considering. To find out the potential difference of this reaction, it is enough to decrease the standard reduction potential of the cathode by that of the anode. This is explained in the text. Potential difference of a battery .
Through the table of standard reduction potentials (E0red), we know that:
At+(ℓ) + and- → In(s) AND0red= -2.71
2Cl-(ℓ) → 2 and- + 1Cl2(g) AND0red= +1.36
Now, just decrease these values to know the potential difference of the global reaction:
∆And0 = AND0red (cathode) - AND0red (anode)
∆And0 = -2,71 – (+ 1,36)
∆And0 = - 4.07 V
Therefore, this means that the cell or battery that will be used must have a voltage equal to or greater than 4.07V to carry out the igneous electrolysis of sodium chloride.
The negative value only indicates that it is a non-spontaneous process.. In the case of batteries, which is a spontaneous process, the electromotive force value (∆E0) always gives positive.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Igneous Sodium Chloride Electrolysis"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/eletrolise-Ignea-cloreto-sodio.htm. Accessed on June 28, 2021.
e) It is a spontaneous redox process.
Chemistry

Applications of Electrolysis, electroplating, nickel plating, chrome plating, nickel, chromium, cathode, sodium, aluminum, chlorine, caustic soda, hydrogen gas, igneous electrolysis, aqueous electrolysis, alkali metals, alkaline earth, gas chlorine.
Chemistry
Electrolysis, electrolyte solutions, electric current, oxidation-reduction reactions, spontaneous chemical process, chemical process non-spontaneous, transformer, artificial transformation, industries, alkali metals, alkaline earth, hydrogen gas, gas cl
