According to French chemist Henry Louis Le Chatelier, equilibrium shift it is a situation in which a chemical reaction is shifted (forward or reverse) when subjected to an external disturbance. This statement proposed by the chemist became known as principle of Le Chatelier.
Note: direct reaction is the one in which the reactants are transformed into products, whereas the inverse reaction is the one in which the products are transformed into reactants.
A reaction is in equilibrium when the speed of the direct reaction is the same as the reverse reaction, that is, the reactants turn into products at the same rate as products turn into reactants.

General representation of an equilibrium chemical reaction
According to Le Chatelier's principle, whenever a force acts on an equilibrium reaction, the equilibrium shift it will take place in order to nullify this disturbance and establish a new equilibrium situation in the reaction.
The disturbances capable of shift a chemical balance they are:
→ concentration variation
when the concentration in quantity of matter (in mol/L or molar) of a participant in the reaction is changed (either decreased or increased), the balance shift, as long as that participant is not in solid state.
Thus, according to Le Chatelier's principle, if the concentration if a participant is increased, the balance will shift in the opposite direction to the increase. However, if the concentration the participant is reduced, there will be a displacement of the balance in the direction of the decrease. For example:

General representation of an equilibrium chemical reaction
Increasing the concentration of A = balance shifts to the right
Decreasing the concentration of B = the balance shifts to the left
→ Variation of pressure
The pressure variation only promotes shift in equilibrium that have gaseous components, since when it is increased the molecules increase the collisions with each other, and when the pressure is decreased, they decrease the collisions with each other.
The collisions between molecules increase with increasing pressure, because the volume (space) is decreased accordingly, while decreasing the pressure is accompanied with increasing volume.
Do not stop now... There's more after the advertising ;)
Note: In a chemical equilibrium, the analysis of the effect of pressure modification takes into account the molar volume of reactants and products, which volume is related to the reaction coefficients. In the equation below, the reagent volume is 4 and the product is 2.
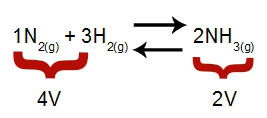
Equation that indicates the volumes present in the formation of ammonia
According to Le Chatelier's principle, if the pressure of a system is increased, the displacement of the balance in the direction of greater volume, while if the pressure is decreased, the displacement of the balance.

Equation representing the balance of ammonia formation
Increasing pressure = balance shifts to the right (because it's the side with less volume).
Decreasing pressure = balance shifts to the left (because this is the higher volume side).
→ Temperature Variation
The increase in temperature of an equilibrium reaction favors the molecules to clash more, while the decrease of temperatura lessens their agitation and, consequently, their shocks. Since the increase in temperature always favors a reaction endothermic (one that absorbs energy).
The analysis of the effect of temperature on an equilibrium mainly takes into account the ΔH of the reaction. If ΔH is positive, the forward reaction will be endothermic, while the reverse will be exothermic. If the ΔH is negative, the right one will be exothermic and the inverse one, endothermic.
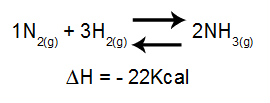
Equation containing enthalpy variation in ammonia formation
Increasing temperature = equilibrium shifts to the left (because this is the direction of the endothermic reaction, since ΔH is negative).
Decreasing temperature = equilibrium shifts to the right (because this is the direction of the exothermic reaction, since ΔH is negative).
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is equilibrium displacement?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-deslocamento-equilibrio.htm. Accessed on June 28, 2021.

