question 1
(FIEB-SP) The inner cup of a calorimeter is made of aluminum and has a mass of 30 g. In its interior, where there is 150 g of pure water at a temperature of 20 °C, 200 g of steel balls are poured, which are initially at a temperature of 60 °C. Knowing that the specific heat of aluminum is 0.2 cal/g.ºC, that of water, 1 cal/g.ºC, and the thermal equilibrium temperature of the set equals 25 °C, the specific heat of the steel and the amount of heat exchanged by the steel balls with the system have values, respectively and approximately, equal to
a) 0.11 cal/g.ºC and 780 cal, provided.
b) 0.11 cal/g.ºC and 780 cal, received.
c) 0.55 cal/g.ºC and 890 cal, provided.
d) 0.55 cal/g.ºC and 890 cal, received.
e) 0.88 cal/g.ºC and 780 cal, provided.
question 2
(UEA-AM) The thermal capacity of a body (C) is defined as the ratio between the amount of heat it receives (Q) and the corresponding temperature variation occurred (ΔT):
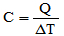
If a body with a thermal capacity of 25 cal/°C receives heat from a source for 20 minutes at a constant rate of 50 cal/min, it undergoes a temperature variation, in °C, equal to
a) 10.0.
b) 40.0.
c) 50.0.
d) 62.5.
e) 84.5.
More questionsWatch the video class and learn how the urbanization theme is found in Enem tests. Understand what urbanization is.
Work is how man modifies nature to meet his needs. See how Enem usually calls for the theme of work and the main characteristics of the Sociology of Work.

